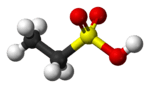
Ethanesulfonic acid
 | |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
ethanesulfonic acid | |||
| Other names
esylic acid, ethylsulfonic acid | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.008.950 | ||
| EC Number | 209-843-0 | ||
PubChem CID |
|||
| UNII | |||
| |||
| |||
| Properties | |||
| C2H6O3S | |||
| Molar mass | 110.13 g·mol−1 | ||
| Appearance | Clear brown liquid | ||
| Density | 1.35 g/mL | ||
| Melting point | −17 °C (1 °F; 256 K) | ||
| Boiling point | 122–123 °C (252–253 °F; 395–396 K) at 0.01mm | ||
| Soluble | |||
| log P | -0.37 | ||
| Acidity (pKa) | -1.68 [1] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
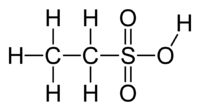
Ethanesulfonic acid (esylic acid) is a sulfonic acid with the chemical formula CH3CH2SO3H. The conjugate base is known as ethanesulfonate or, when used in pharmaceutical formulations, as esilate.[2]
References
- ↑ YK Ye & RW Stringham (2001-06-21). ""Effect of mobile phase acidic additives on enantioselectivity for phenylalanine analogs"" (PDF). J Chromatogr A.
- ↑ "Ethanesulfonate". ChemSpider.
- ↑ "FDA Substance Registration System". fdasis.nlm.nih.gov. Retrieved 2016-09-02.
- ↑ "Ethanesulfonic acid(594-45-6) MSDS Melting Point Boiling Point Density Storage Transport". www.chemicalbook.com. Retrieved 2016-09-03.
- ↑ DrugBank, ed. (2016-08-17). "Ethanesulfonic Acid". DrugBank.
- ↑ "Ethanesulfonic acid | C2H6O3S | ChemSpider". www.chemspider.com. Retrieved 2016-09-02.
- ↑ EtSO3H, pKa (water) −1.68. Tully PS. Sulfonic Acids. In: Kroschwitz, editor. Kirk-Othmer Encyclopedia of Chemical Technology. 4th Ed. Vol. 23. Wiley; New York: 1997. pp. 194. ISBN 9780471238966
External links
- Ye YK, Stringham RW (2006), The effect of acidic and basic additives on the enantioseparation of basic drugs using polysaccharide-based chiral stationary phases. Chirality 18, 519-530. (PubMed:16676332)
- National Center for Biotechnology Information (2005), Ethanesulfonic acid, PubChem Compound Database; CID=11668, (accessed December 23, 2016)
- https://www.scbt.com/scbt/product/ethanesulfonic-acid-594-45-6
- http://www.chemicalbook.com/productmsdsdetailcb5173859_en.htm
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.