Erlenmeyer–Plöchl azlactone and amino-acid synthesis
The Erlenmeyer–Plöchl azlactone and amino acid synthesis, named after Friedrich Gustav Carl Emil Erlenmeyer who partly discovered the reaction, is a series of chemical reactions which transform an N-acyl glycine to various other amino acids via an oxazolone and an azlactone.[1][2]
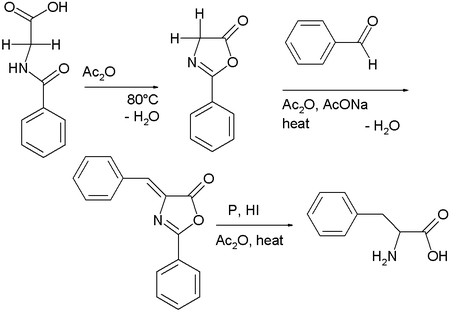
Hippuric acid, the benzamide derivative of glycine, cyclizes in the presence of acetic anhydride, condensing to give 2-phenyl-oxazolone.[3] This intermediate also has two acidic protons and reacts with benzaldehyde, acetic anhydride and sodium acetate to a so-called azlactone. This compound on reduction gives access to phenylalanine.[4]
Variations
Variants of the azlactone synthesis in which analogues of azlactones are used are sometimes advantageous. Hydantoin (in Bergmann modification), thiohydantoin and rhodanine have each been employed as the enolate-forming component of the condensation. [5][6]
Scope
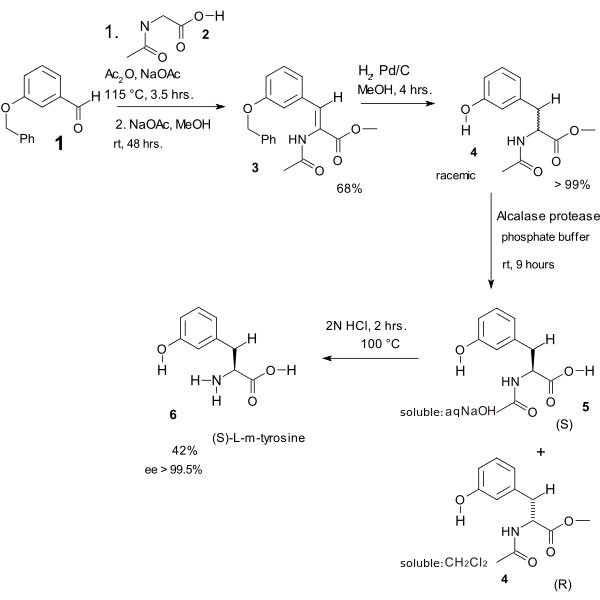
In one study the Erlenmeyer amino acid synthesis was used in the heart of an L-m-tyrosine synthesis [7][8]
See also
References
- ↑ Plöchl, J. (1884). "Über einige Derivate der Benzoylimidozimtsäure" [On some derivatives of benzoyl-imido-cinnaminic acid]. Berichte der deutschen chemischen Gesellschaft. 17: 1616–1624. ; see especially pp. 1623-1624.
- ↑ Erlenmeyer, F. (1893). "Ueber die Condensation der Hippursäure mit Phtalsäureanhydrid und mit Benzaldehyd" [On the condensation of hippuric acid with phthalic acid anhydride and with benzaldehyde]. Annalen der Chemie. 275: 1–8. doi:10.1002/jlac.18932750102. ; see especially pp. 3-8.
- ↑ G. E. VandenBerg, J. B. Harrison, H. E. Carter, B. J. Magerlein (1973). "2-Phenyl-2-oxazolone". Organic Syntheses. ; Collective Volume, 5, p. 946
- ↑ H. B. Gillespie, H. R. Snyder (1934). "dl-β-Phenylalanine". Organic Syntheses. ; Collective Volume, 2, p. 489
- ↑ Alfred Hassner, Irishi Namboothiri (2011). Organic Syntheses Based on Name Reactions: A Practical Guide to 750 Transformations. Elsevier. p. 139.
- ↑ Richard O.C. Norman, James M. Coxon (1993). Principles of Organic Synthesis (3rd ed.). CRC Press. p. 219–220.
- ↑ Cara E. Humphrey, Markus Furegati, Kurt Laumen, Luigi La Vecchia, Thomas Leutert, J. Constanze D. Müller-Hartwieg, and Markus Vögtle (2007). "Optimized Synthesis of L-m-Tyrosine Suitable for Chemical Scale-Up". Organic Process Research & Development. 11 (6): 1069–1075. doi:10.1021/op700093y.
- ↑ The benzyl ether of 3-hydroxybenzaldehyde 1 reacts with the N-acetyl amide of glycine 2, acetic anhydride and sodium acetate to the azlactone (not displayed) which is ring-opened with sodium acetate in methanol to dehydroamino acid 3. Hydrogenation gives the N-acyl-m-tyrosine methyl ester 4 (the benzyl ether group is also cleaved). This compound is racemic and kinetic resolution is brought about by an enzyme which is able to only cleave the methyl ester of the S-enantiomer (forming (S)-5 soluble in dichloromethane) leaving water soluble (R)-4 untouched. The final step is amide cleavage to (S)-L-m-tyrosine 6