Electrostatics


Electrostatics is a branch of physics that studies electric charges at rest.
Since classical physics, it has been known that some materials such as amber attract lightweight particles after rubbing. The Greek word for amber, ήλεκτρον, or electron, was the source of the word 'electricity'. Electrostatic phenomena arise from the forces that electric charges exert on each other. Such forces are described by Coulomb's law. Even though electrostatically induced forces seem to be rather weak, some electrostatic forces such as the one between an electron and a proton, that together make up a hydrogen atom, is about 36 orders of magnitude stronger than the gravitational force acting between them.
There are many examples of electrostatic phenomena, from those as simple as the attraction of the plastic wrap to one's hand after it is removed from a package to the apparently spontaneous explosion of grain silos, the damage of electronic components during manufacturing, and photocopier & laser printer operation. Electrostatics involves the buildup of charge on the surface of objects due to contact with other surfaces. Although charge exchange happens whenever any two surfaces contact and separate, the effects of charge exchange are usually only noticed when at least one of the surfaces has a high resistance to electrical flow. This is because the charges that transfer are trapped there for a time long enough for their effects to be observed. These charges then remain on the object until they either bleed off to ground or are quickly neutralized by a discharge: e.g., the familiar phenomenon of a static 'shock' is caused by the neutralization of charge built up in the body from contact with insulated surfaces.
Coulomb's law
Coulomb's law states that:
'The magnitude of the electrostatic force of attraction or repulsion between two point charges is directly proportional to the product of the magnitudes of charges and inversely proportional to the square of the distance between them.'
The force is along the straight line joining them. If the two charges have the same sign, the electrostatic force between them is repulsive; if they have different signs, the force between them is attractive.
If is the distance (in meters) between two charges, then the force (in newtons) between two point charges and (in coulombs) is:
where ε0 is the vacuum permittivity, or permittivity of free space:[1]
The SI units of ε0 are equivalently A2s4 kg−1m−3 or C2N−1m−2 or F m−1. Coulomb's constant is:
The use of ε0 instead of k0 in expressing Coulomb's Law is related to the fact that the force is inversely proportional to the surface area of a sphere with radius equal to the separation between the two charges.
A single proton has a charge of e, and the electron has a charge of −e, where,
These physical constants (ε0, k0, e) are currently defined so that ε0 and k0 are exactly defined, and e is a measured quantity.
Electric field
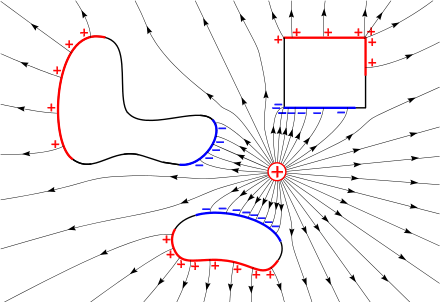
The electric field, , in units of newtons per coulomb or volts per meter, is a vector field that can be defined everywhere, except at the location of point charges (where it diverges to infinity).[2] It is defined as the electrostatic force in newtons on a hypothetical small test charge at the point due to Coulomb's Law, divided by the magnitude of the charge in coulombs
Electric field lines are useful for visualizing the electric field. Field lines begin on positive charge and terminate on negative charge. They are parallel to the direction of the electric field at each point, and the density of these field lines is a measure of the magnitude of the electric field at any given point.
Consider a collection of particles of charge , located at points (called source points), the electric field at (called the field point) is:[2]
where is the displacement vector from a source point to the field point , and is a unit vector that indicates the direction of the field. For a single point charge at the origin, the magnitude of this electric field is and points away from that charge is positive. The fact that the force (and hence the field) can be calculated by summing over all the contributions due to individual source particles is an example of the superposition principle. The electric field produced by a distribution of charges is given by the volume charge density and can be obtained by converting this sum into a triple integral:
Gauss' law
Gauss' law states that "the total electric flux through any closed surface in free space of any shape drawn in an electric field is proportional to the total electric charge enclosed by the surface." Mathematically, Gauss's law takes the form of an integral equation:
where is a volume element. If the charge is distributed over a surface or along a line, replace by or . The Divergence Theorem allows Gauss's Law to be written in differential form:
where is the divergence operator.
Poisson and Laplace equations
The definition of electrostatic potential, combined with the differential form of Gauss's law (above), provides a relationship between the potential Φ and the charge density ρ:
This relationship is a form of Poisson's equation. In the absence of unpaired electric charge, the equation becomes Laplace's equation:
Electrostatic approximation
The validity of the electrostatic approximation rests on the assumption that the electric field is irrotational:
From Faraday's law, this assumption implies the absence or near-absence of time-varying magnetic fields:
In other words, electrostatics does not require the absence of magnetic fields or electric currents. Rather, if magnetic fields or electric currents do exist, they must not change with time, or in the worst-case, they must change with time only very slowly. In some problems, both electrostatics and magnetostatics may be required for accurate predictions, but the coupling between the two can still be ignored. Electrostatics and magnetostatics can both be seen as Galilean limits for electromagnetism.[3]
Electrostatic potential
Because the electric field is irrotational, it is possible to express the electric field as the gradient of a scalar function, , called the electrostatic potential (also known as the voltage). An electric field, , points from regions of high electric potential to regions of low electric potential, expressed mathematically as
The gradient theorem can be used to establish that the electrostatic potential is the amount of work per unit charge required to move a charge from point to point with the following line integral:
From these equations, we see that the electric potential is constant in any region for which the electric field vanishes (such as occurs inside a conducting object).
Electrostatic energy
A single test particle's potential energy, , can be calculated from a line integral of the work, . We integrate from a point at infinity, and assume a collection of particles of charge , are already situated at the points . This potential energy (in Joules) is:
where is the distance of each charge from the test charge , which situated at the point , and is the electric potential that would be at if the test charge were not present. If only two charges are present, the potential energy is . The total electric potential energy due a collection of N charges is calculating by assembling these particles one at a time:
where the following sum from, j = 1 to N, excludes i = j:
This electric potential, is what would be measured at if the charge were missing. This formula obviously excludes the (infinite) energy that would be required to assemble each point charge from a disperse cloud of charge. The sum over charges can be converted into an integral over charge density using the prescription :
- ,
This second expression for electrostatic energy uses the fact that the electric field is the negative gradient of the electric potential, as well as vector calculus identities in a way that resembles integration by parts. These two integrals for electric field energy seem to indicate two mutually exclusive formulas for electrostatic energy density, namely and ; they yield equal values for the total electrostatic energy only if both are integrated over all space.
Electrostatic pressure
On a conductor, a surface charge will experience a force in the presence of an electric field. This force is the average of the discontinuous electric field at the surface charge. This average in terms of the field just outside the surface amounts to:
- ,
This pressure tends to draw the conductor into the field, regardless of the sign of the surface charge.
Triboelectric series
The triboelectric effect is a type of contact electrification in which certain materials become electrically charged when they are brought into contact with a different material and then separated. One of the materials acquires a positive charge, and the other acquires an equal negative charge. The polarity and strength of the charges produced differ according to the materials, surface roughness, temperature, strain, and other properties. Amber, for example, can acquire an electric charge by friction with a material like wool. This property, first recorded by Thales of Miletus, was the first electrical phenomenon investigated by humans. Other examples of materials that can acquire a significant charge when rubbed together include glass rubbed with silk, and hard rubber rubbed with fur.
Electrostatic generators
The presence of surface charge imbalance means that the objects will exhibit attractive or repulsive forces. This surface charge imbalance, which yields static electricity, can be generated by touching two differing surfaces together and then separating them due to the phenomena of contact electrification and the triboelectric effect. Rubbing two nonconductive objects generates a great amount of static electricity. This is not just the result of friction; two nonconductive surfaces can become charged by just being placed one on top of the other. Since most surfaces have a rough texture, it takes longer to achieve charging through contact than through rubbing. Rubbing objects together increases amount of adhesive contact between the two surfaces. Usually insulators, e.g., substances that do not conduct electricity, are good at both generating, and holding, a surface charge. Some examples of these substances are rubber, plastic, glass, and pith. Conductive objects only rarely generate charge imbalance except, for example, when a metal surface is impacted by solid or liquid nonconductors. The charge that is transferred during contact electrification is stored on the surface of each object. Static electric generators, devices which produce very high voltage at very low current and used for classroom physics demonstrations, rely on this effect.
Note that the presence of electric current does not detract from the electrostatic forces nor from the sparking, from the corona discharge, or other phenomena. Both phenomena can exist simultaneously in the same system.
- See also: Friction machines, Wimshurst machines, and Van de Graaff generators.
Charge neutralization
Natural electrostatic phenomena are most familiar as an occasional annoyance in seasons of low humidity, but can be destructive and harmful in some situations (e.g. electronics manufacturing). When working in direct contact with integrated circuit electronics (especially delicate MOSFETs), or in the presence of flammable gas, care must be taken to avoid accumulating and suddenly discharging a static charge (see electrostatic discharge).
Electrostatic induction
Electrostatic induction, discovered by British scientist John Canton in 1753 and Swedish professor Johan Carl Wilcke in 1762[4][5][6] is a redistribution of charges in an object caused by the electric field of a nearby charge. For example, if a positively charged object is brought near an uncharged metal object, the mobile negatively-charged electrons in the metal will be attracted the external charge, and move to the side of the metal facing it, creating a negative charge on the surface. When the electrons move out of an area they leave a positive charge due to the metal atoms' nuclei, so the side of the metal object facing away from the charge acquires a positive charge. These induced charges disappear when the external charge is removed. Induction is also responsible for the attraction of light objects, such as balloons, paper scraps and styrofoam packing peanuts to static charges. The surface charges induced in conductive objects exactly cancel external electric fields inside the conductor, so there is no electric field inside a metal object. This is the basis for the electric field shielding action of a Faraday cage. Since the electric field is the gradient of the voltage, electrostatic induction is also responsible for making the electric potential (voltage) constant throughout a conductive object.
'Static' electricity
Before the year 1832, when Michael Faraday published the results of his experiment on the identity of electricities, physicists thought "static electricity" was somehow different from other electrical charges. Michael Faraday proved that the electricity induced from the magnet, voltaic electricity produced by a battery, and static electricity are all the same.
Static electricity is usually caused when certain materials are rubbed against each other, like wool on plastic or the soles of shoes on carpet. The process causes electrons to be pulled from the surface of one material and relocated on the surface of the other material.
A static shock occurs when the surface of the second material, negatively charged with electrons, touches a positively charged conductor, or vice versa.
Static electricity is commonly used in xerography, air filters, and some automotive paints. Static electricity is a buildup of electric charges on two objects that have become separated from each other. Small electrical components can easily be damaged by static electricity. Component manufacturers use a number of antistatic devices to avoid this.
Static electricity and chemical industry
When different materials are brought together and then separated, an accumulation of electric charge can occur which leaves one material positively charged while the other becomes negatively charged. The mild shock that you receive when touching a grounded object after walking on carpet is an example of excess electrical charge accumulating in your body from frictional charging between your shoes and the carpet. The resulting charge build-up upon your body can generate a strong electrical discharge. Although experimenting with static electricity may be fun, similar sparks create severe hazards in those industries dealing with flammable substances, where a small electrical spark may ignite explosive mixtures with devastating consequences.
A similar charging mechanism can occur within low conductivity fluids flowing through pipelines—a process called flow electrification. Fluids which have low electrical conductivity (below 50 picosiemens per meter), are called accumulators. Fluids having conductivities above 50 pS/m are called non-accumulators. In non-accumulators, charges recombine as fast as they are separated and hence electrostatic charge generation is not significant. In the petrochemical industry, 50 pS/m is the recommended minimum value of electrical conductivity for adequate removal of charge from a fluid.
An important concept for insulating fluids is the static relaxation time. This is similar to the time constant (tau) within an RC circuit. For insulating materials, it is the ratio of the static dielectric constant divided by the electrical conductivity of the material. For hydrocarbon fluids, this is sometimes approximated by dividing the number 18 by the electrical conductivity of the fluid. Thus a fluid that has an electrical conductivity of 1 pS/cm (100 pS/m) will have an estimated relaxation time of about 18 seconds. The excess charge within a fluid will be almost completely dissipated after 4 to 5 times the relaxation time, or 90 seconds for the fluid in the above example.
Charge generation increases at higher fluid velocities and larger pipe diameters, becoming quite significant in pipes 8 inches (200 mm) or larger. Static charge generation in these systems is best controlled by limiting fluid velocity. The British standard BS PD CLC/TR 50404:2003 (formerly BS-5958-Part 2) Code of Practice for Control of Undesirable Static Electricity prescribes velocity limits. Because of its large impact on dielectric constant, the recommended velocity for hydrocarbon fluids containing water should be limited to 1 m/s.
Bonding and earthing are the usual ways by which charge buildup can be prevented. For fluids with electrical conductivity below 10 pS/m, bonding and earthing are not adequate for charge dissipation, and anti-static additives may be required.
Applicable standards
1.BS PD CLC/TR 50404:2003 Code of Practice for Control of Undesirable Static Electricity
2.NFPA 77 (2007) Recommended Practice on Static Electricity
3.API RP 2003 (1998) Protection Against Ignitions Arising Out of Static, Lightning, and Stray Currents
Electrostatic induction in commercial applications
Electrostatic induction was used in the past to build high-voltage generators known as Influence machines. The main component that emerged in these times is the capacitor. Electrostatic induction is also used for electro-mechanic precipitation or projection.In such technologies, charged particles of small sizes are collected or deposited intentionally on surfaces. Applications range from Electrostatic precipitator to Spray painting or Inkjet printing. Recently a new Wireless power Transfer Technology has been based on electrostatic induction between oscillating distant dipoles.
See also
References
- Faraday, Michael (1839). Experimental Researches in Electricity. London: Royal Inst.
- e-book at Project Gutenberg
- Halliday, David; Robert Resnick; Kenneth S. Krane (1992). Physics. New York: John Wiley & Sons. ISBN 0-471-80457-6.
- Griffiths, David J. (1999). Introduction to Electrodynamics. Upper Saddle River, NJ: Prentice Hall. ISBN 0-13-805326-X.
- Hermann A. Haus; James R. Melcher (1989). Electromagnetic Fields and Energy. Englewood Cliffs, NJ: Prentice-Hall. ISBN 0-13-249020-X.
- ↑ Matthew Sadiku (2009). Elements of electromagnetics. p. 104. ISBN 9780195387759.
- 1 2 Purcell, Edward M. (2013). Electricity and Magnetism. Cambridge University Press. pp. 16–18. ISBN 1107014026.
- ↑ Heras, J. A. (2010). "The Galilean limits of Maxwell's equations". American Journal of Physics. 78 (10): 1048. arXiv:1012.1068. Bibcode:2010AmJPh..78.1048H. doi:10.1119/1.3442798.
- ↑ "Electricity". Encyclopaedia Britannica, 11th Ed. 9. The Encyclopaedia Britannica Co. 1910. p. 181. Retrieved 2008-06-23.
- ↑ Heilbron, J. L. (1979). Electricity in the 17th and 18th Centuries: A Study of Early Modern Physics. Univ. of California Press. ISBN 0520034783.
- ↑ Sarkar, T. K.; Mailloux, Robert; Oliner, Arthur A., Ed. (2006). History of Wireless. John Wiley and Sons. p. 9. ISBN 0471783013.
Further reading
- Essays
- William J. Beaty, "Humans and sparks; The Cause, Stopping the Pain, and 'Electric People". 1997.
- Books
- William Cecil Dampier, "The theory of experimental electricity". Cambridge [Eng.] University press, 1905 (Cambridge physical series). xi, 334 p. illus., diagrs. 23 cm. LCCN 05040419 //r33
- William Thomson Kelvin, Reprint of Papers on Electrostatics and Magnetism By William Thomson Kelvin, Macmillan 1872
- Alexander MacAulay Utility of Quaternions in Physics. Electrostatics—General Problem. Macmillan 1893
- Alexander Russell, A Treatise on the Theory of Alternating Currents. Electrostatics. University Press 1904
External links
| Look up electrostatics in Wiktionary, the free dictionary. |
- Man's static jacket sparks alert". BBC News, 16 September 2005.
- Static Electricity and Plastics
- "Can shocks from static electricity damage your health?". Wolfson Electrostatics News pages.
- Invisible wall of static:
- Downloadable electrostatic BEM modules in MATLAB for simple capacitance problems
- Introduction to Electrostatics: Point charges can be treated as a distribution using the Dirac delta function
| Library resources about Electrostatics |
![]()

