Diphenylchlorarsine
 | |
 | |
| Names | |
|---|---|
| IUPAC name
diphenylchloroarsenic, chlorodiphenylarsane, sneezing gas | |
| Other names
diphenylchlorarsine | |
| Identifiers | |
3D model (JSmol) |
|
| Abbreviations | Ph2AsCl |
| ChemSpider | |
| ECHA InfoCard | 100.010.839 |
PubChem CID |
|
| |
| |
| Properties | |
| C12H10AsCl | |
| Molar mass | 264.59 g mol−1 |
| Appearance | colorless crystalline solid |
| Density | 1.55 g/cm3 |
| Melting point | 42 °C (108 °F; 315 K) |
| -145.5·10−6 cm3/mol | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
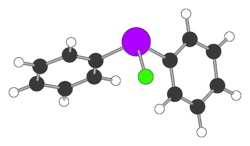
Diphenylchloroarsine (DA) is the organoarsenic compound with the formula (C6H5)2AsCl. It is highly toxic and was once used in chemical warfare. It is also an intermediate in the preparation of other organoarsenic compounds. The molecule consists of a pyramidal As(III) center attached to two phenyl rings and one chloride.
Preparation and structure
It was first produced in 1878 by the German chemists August Michaelis (1847–1916) and Wilhelm La Coste (1854–1885).[1][2] It is prepared by the reduction of diphenylarsinic acid with sulfur dioxide. An idealized equation is shown:[3]
- Ph2AsO2H + SO2 + HCl → Ph2AsCl + H2O + SO3
The structure consists of pyramidal As centre. The As-Cl distance is 2.26 A and the Cl-As-C and C-As-C angles are 96 and 105°, respectively.[4]
Uses
It is a useful reagent for the preparation of other diphenylarsenic compounds, e.g. by reactions with Grignard reagents:
Chemical warfare
Diphenylchlorarsine was used as a chemical weapon on the Western front during the trench warfare of World War I.[5] It belongs to the class of chemicals classified as vomiting agents. Other such agents are diphenylcyanoarsine (DC) and diphenylaminechlorarsine (DM, adamsite).[6] Diphenylchlorarsine was sometimes believed to penetrate the gas masks of the time and to cause violent sneezing, forcing removal of the protecting device. The Germans called it Maskenbrecher (mask breaker), together with other substances with similar effects, such as Adamsite, diphenylarsincyanide, and diphenylaminarsincyanide. This gas did not actually penetrate masks any better than other gases.[7]
Safety
Diphenylchlorarsine is known to cause sneezing, coughing, headache, salivation, and vomiting. China and Japan are negotiating remediation of stocks of a variety of organoarsenic weapons dumped in northeastern China after Japan's numerous invasions of China, including chlorodiphenylarsine.[8]
See also
References
- ↑ As early as 1875, Michaelis found diphenylchlorarsine as a side-product in a reaction to produce monophenylarsenic chloride: Michaelis, A. (1875). "Ueber aromatische Arsenverbindungen" [On aromatic arsenic compounds]. Berichte der Deutschen Chemischen Gesellschaft (in German). 8: 1316–1317. From p. 1317: "Der zweite neben Phenylarsenchlorid gebildete weisse Körper ist noch nicht untersucht, er ist wahrscheinlich Diphenylarsenchlorid, … " (The second white substance that formed beside phenylarsenic chloride has still not been investigated, it's probably diphenylarsenic chloride, … ) In 1878, Michaelis confirmed that it was indeed diphenylarsenic chloride.(La Coste & Michaelis, 1878), p. 1885
- ↑ La Coste, W.; Michaelis, A. (1878). "Ueber Mono- und Diphenylarsenverbindungen" [On mono- and di-phenyl arsenic compounds]. Berichte der Deutschen Chemischen Gesellschaft (in German). 11: 1883–1887. See Diphenylarsenchlorür on p. 1885.
- ↑ Blicke, F. F.; Smith, F. D. (1929). "Action of Aromatic Grignard Reagents on Arsenic Trioxide". Journal of the American Chemical Society. 51 (5): 1558–1565. doi:10.1021/ja01380a038.
- ↑ Trotter, J. (1962). "Stereochemistry of Arsenic. IV. Chlorodiphenylarsine". Canadian Journal of Chemistry. 40 (8): 1590–1593. doi:10.1139/v62-241.
- ↑ Gilbert, M. (1995). The First World War—A Complete History. HarperCollins. ISBN 0805047344.
- ↑ Holstege, C. P.; Boyle, J. S. (2008-11-26). "CBRNE - Vomiting Agents - Dm, Da, Dc". Medscape.
- ↑ Gas and Flame in Modern Warfare, 1918, Chapter IX.
- ↑ "Abandoned Chemical Weapons (ACW) in China". 02/06/2004. Archived from the original on July 4, 2008. Check date values in:
|date=(help)