Rankine scale
| from Rankine | to Rankine | |
|---|---|---|
| Celsius | [°C] = ([°R] − 491.67) × 5⁄9 | [°R] = ([°C] + 273.15) × 9⁄5 |
| Fahrenheit | [°F] = [°R] − 459.67 | [°R] = [°F] + 459.67 |
| Kelvin | [K] = [°R] × 5⁄9 | [°R] = [K] × 9⁄5 |
| For temperature intervals rather than specific temperatures, 1 °R = 1 °F = 5⁄9 °C = 5⁄9 K Comparisons among various temperature scales | ||
The Rankine scale (/ˈræŋkɪn/) is an absolute scale of thermodynamic temperature named after the Glasgow University engineer and physicist William John Macquorn Rankine, who proposed it in 1859. (The Kelvin scale was first proposed in 1848.)[1] It may be used in engineering systems where heat computations are done using degrees Fahrenheit.
The symbol for degrees Rankine is °R[2] (or °Ra if necessary to distinguish it from the Rømer and Réaumur scales). By analogy with kelvin, some authors term the unit rankine, omitting the degree symbol.[3][4] Zero on both the Kelvin and Rankine scales is absolute zero, but a temperature difference of one Rankine degree is defined as equal to one Fahrenheit degree, rather than the Celsius degree used on the Kelvin scale. Thus, a temperature of 0 K (−273.15 °C; −459.67 °F) is equal to 0 °R, and a temperature of −458.67 °F equal to 1 °R.
The US National Institute of Standards and Technology recommends against using the degree symbol when using Rankine in NIST publications.[2]
Some important temperatures relating the Rankine scale to other temperature scales are shown in the table below.
| Kelvin | Celsius | Fahrenheit | Rankine | |
|---|---|---|---|---|
| Absolute zero (by definition) |
0 K | −273.15 °C | −459.67 °F | 0 °R |
| Freezing point of brine (zero point of Fahrenheit scale, old definition) |
255.37 K | −17.78 °C | 0 °F | 459.67 °R |
| Freezing point of water[5] | 273.15 K | 0 °C | 32 °F | 491.67 °R |
| Triple point of water (by definition) |
273.16 K | 0.01 °C | 32.018 °F | 491.688 °R |
| Boiling point of water[6] | 373.1339 K | 99.9839 °C | 211.97102 °F | 671.64102 °R |
Conversion table between the temperature units
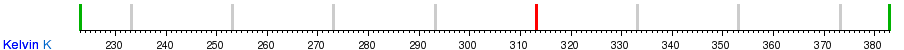
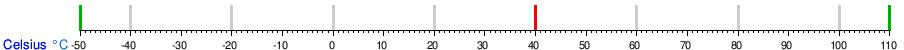
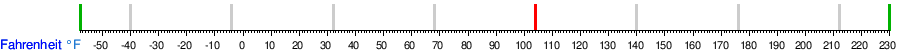

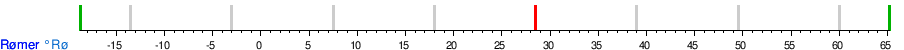



See also
Notes and references
- ↑ http://www.merriam-webster.com/dictionary/rankine
- 1 2 B.8 Factors for Units Listed Alphabetically from Guide for the Use of the International System of Units (SI), NIST Special Publication 811, 2008 edition, Ambler Thompson and Barry N. Taylor
- ↑ Pauken, Michael (2011). Thermodynamics For Dummies. Indianapolis: Wiley Publishing Inc. p. 20. ISBN 978-1-118-00291-9.
- ↑ Balmer, Robert (2011). Modern Engineering Thermodynamics. Oxford: Elsevier Inc. p. 10. ISBN 978-0-12-374996-3.
- ↑ The ice point of purified water has been measured to be 0.000089(10) degrees Celsius – see Magnum, B.W. (June 1995). "Reproducibility of the Temperature of the Ice Point in Routine Measurements" (PDF). Nist Technical Note. 1411. Archived from the original (PDF) on 2007-03-07. Retrieved 2007-02-11.
- ↑ For Vienna Standard Mean Ocean Water at one standard atmosphere (101.325 kPa) when calibrated solely per the two-point definition of thermodynamic temperature. Older definitions of the Celsius scale once defined the boiling point of water under one standard atmosphere as being precisely 100 °C. However, the current definition results in a boiling point that is actually 16.1 mK less. For more about the actual boiling point of water, see VSMOW in temperature measurement.
External links
| Look up degree Rankine in Wiktionary, the free dictionary. |