Degree of unsaturation
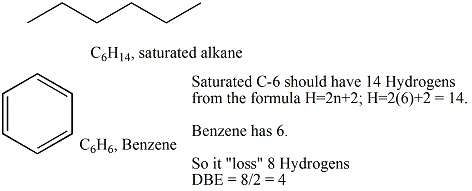
In the analysis of the molecular formula of organic molecules, the degree of unsaturation (also known as the index of hydrogen deficiency (IHD), double bond equivalents, or unsaturation index[1]) is a calculation that determines the total number of rings and π bonds. formula is used in organic chemistry to help draw chemical structures. It does not give any information about those components individually—the specific number of rings, or of double bonds (one π bond each), or of triple bonds (two π bonds each). The final structure is verified with use of NMR, mass spectrometry and IR spectroscopy, as well as qualitative inspection. It is based on comparing the actual molecular formula to what would be a possible formula if the structure were saturated—having no rings and containing only σ bonds—with all atoms having their standard valence.
General formula
The formula for degree of unsaturation is:
where ni is the number of atoms with valence vi.[2]
That is, an atom that has a valence of x contributes a total of x − 2 to the degree of unsaturation. The result is then halved and increased by 1.
Simplified formulas
For certain classes of molecules, the general formula can be simplified or rewritten more clearly. For example:
where
- a = number of carbon atoms in the compound
- b = number of hydrogen atoms in the compound
- c = number of nitrogen atoms in the compound
- f = number of halogen atoms in the compound
or
where C = number of carbons, H = number of hydrogens, X = number of halogens and N = number of nitrogens,[3] gives an equivalent result.
In either case, oxygen and other divalent atoms do not contribute to the degree of unsaturation, as 2 − 2 = 0.
References
- ↑ Sparkman, David O. Mass Spectrometry Desk Reference. Pittsburgh: Global View Pub. p. 54. ISBN 0-9660813-9-0.
- ↑ Badertscher, M.; Bischofberger, K.; Munk, M.E.; Pretsch, E. (2001). "A Novel Formalism To Characterize the Degree of Unsaturation of Organic Molecules". Journal of Chemical Information and Modeling. 41 (4): 889. doi:10.1021/ci000135o.
- ↑ Organic structural spectroscopy, chapter 1.
Young, Paul R. Practical Spectroscopy. ISBN 0-534-37230-9.