Cyclic alkyl amino carbenes
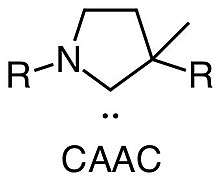
In chemistry, cyclic alkyl amino carbenes (CAACs) are a type of cyclic carbene which contain a single nitrogen atom within the cyclic structure.[1] CAACs were first developed by Guy Bertrand at UCSD as an alternative to traditional N-heterocyclic carbenes which contain two nitrogen atoms with in the structure typically derived from imidazole. Replacing a single nitrogen in the NHC to a quaternary carbon in the CAAC makes a difference to the electron donating abilities of the carbene hence making it a stronger π-acceptor as well as σ-donor ligand.[2]
Current research is investigating the use of these ligands in organometallic compounds as catalysts[3] and into developing new derivatives of CAACs such as bicyclic CAACs.[4]
CAAC synthesis
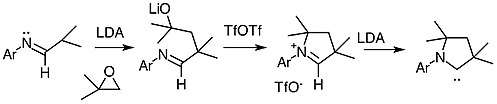
Cyclic alkyl amino carbenes can be prepared from 2,6-disopropylaniline and 2-methylpropanal. The initial step involves the deprotonation of 2,6-disopropylaniline with lithium diisopropylamide to give a aza-allyl anion. The aza-allyl anion then induces the ring opening of 1,2-epoxy-2-methylpro-pane leading to a lithium alkoxide. The lithium alkoxide is then treated with triflic anhydride at -78 celsius, this is then warmed to room temperature to generate the aldiminium salt. The production of the aldiminium salt gives a yield of 58% with respect to 2,6-disopropylaniline. The final step in the synthesis uses lithium diisopropylamide to generate the carbene which is a white solid.[1]
References
- 1 2 Lavallo, Vincent; Canac, Yves; Präsang, Carsten; Donnadieu, Bruno; Bertrand, Guy (2005-09-05). "Stable Cyclic (Alkyl)(Amino)Carbenes as Rigid or Flexible, Bulky, Electron-Rich Ligands for Transition-Metal Catalysts: A Quaternary Carbon Atom Makes the Difference". Angewandte Chemie International Edition. 44 (35): 5705–5709. doi:10.1002/anie.200501841. ISSN 1521-3773. PMC 2427276. PMID 16059961.
- ↑ "Research Highlights". bertrandgroup.ucsd.edu. Retrieved 2017-07-13.
- ↑ Romero, Erik A.; Jazzar, Rodolphe; Bertrand, Guy (2017-02-01). "(CAAC)CuX-catalyzed hydroboration of terminal alkynes with pinacolborane directed by the X-ligand". Journal of Organometallic Chemistry. Frontiers in Organometallic Chemistry. 829: 11–13. doi:10.1016/j.jorganchem.2016.09.025.
- ↑ Tomás-Mendivil, Eder; Hansmann, Max M.; Weinstein, Cory M.; Jazzar, Rodolphe; Melaimi, Mohand; Bertrand, Guy (2017-06-14). "Bicyclic (Alkyl)(amino)carbenes (BICAACs): Stable Carbenes More Ambiphilic than CAACs". Journal of the American Chemical Society. 139 (23): 7753–7756. doi:10.1021/jacs.7b04640. ISSN 0002-7863. PMID 28541687.