Cupferron
 | |
| Names | |
|---|---|
| Other names
cupferron ammonium N-nitrosophenylhydroxylamine | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.004.713 |
PubChem CID |
|
| |
| |
| Properties | |
| C6H9N3O2 | |
| Molar mass | 155.15 g/mol |
| Melting point | 150 to 155 °C (302 to 311 °F; 423 to 428 K) |
| soluble | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
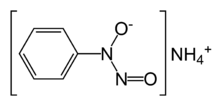
Cupferron, the ammonium salt of N-nitroso-N-phenylhydroxylamine, is a common reagent for the complexation of metal ions. Its formula is NH4[C6H5N(O)NO]. The anion binds to metal cations through the two oxygen atoms, forming five-membered chelate rings.
Cupferron is prepared from phenylhydroxylamine and an NO+ source:
- C6H5NHOH + C4H9ONO + NH3 → NH4[C6H5N(O)NO] + C4H9OH
References
- C. S. Marvel "Cupferron" Organic Syntheses, Coll. Vol. 1, p. 177; Vol. 4, p. 19.
- D. Van der Helm, L. L. Merritt Jnr, R. Degeilh and C. H. MacGillavry "The crystal structure of iron cupferron Fe(O2N2C6H5)3" Acta Crystallogr. (1965). 18, 355–362
- Merck 13,2649; Beil. 16,IV,891
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.