Covalent superconductor
Covalent superconductors are superconducting materials where the atoms are linked by covalent bonds. The first such material was boron-doped synthetic diamond grown by the high-pressure high-temperature (HPHT) method.[1] The discovery had no practical importance, but surprised most scientists as superconductivity had not been observed in covalent semiconductors, including diamond and silicon.
History
The priority of many discoveries in science is vigorously disputed (see, e.g., Nobel Prize controversies). Another example, after Sumio Iijima has "discovered" carbon nanotubes in 1991, many scientists have pointed out that carbon nanofibers were actually observed decades earlier. The same could be said about superconductivity in covalent semiconductors. Superconductivity in germanium and silicon-germanium was predicted theoretically as early as in the 1960s.[2][3] Shortly after, superconductivity was experimentally detected in germanium telluride.[4][5] In 1976, superconductivity with Tc = 3.5 K was observed experimentally in germanium implanted with copper ions;[6] it was experimentally demonstrated that amorphization was essential for the superconductivity (in Ge), and the superconductivity was assigned to Ge itself, not copper.
Diamond
Superconductivity in diamond was achieved through heavy p-type doping by boron such that the individual doping atoms started interacting and formed an "impurity band". The superconductivity was of type-II with the critical temperature Tc = 4 K and critical magnetic field Hc = 4 T. Later, Tc ~ 11K has been achieved in homoepitaxial CVD films.[7][8]
Carbon nanotubes
While there have been reports of intrinsic superconductivity in carbon nanotubes,[9][10] many other experiments found no evidence of superconductivity, and the validity of these results remains a subject of debate.[11] Note, however, a crucial difference between nanotubes and diamond: Although nanotubes contain covalently bonded carbon atoms, they are closer in properties to graphite than diamond, and can be metallic without doping. Meanwhile, undoped diamond is an insulator.
Intercalated graphite
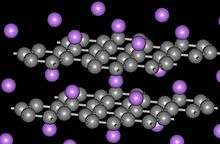
When metal atoms are inserted (intercalated) between the graphite planes, several superconductors are created with the following transition temperatures:[12][13]
| Material | CaC6 | Li3Ca2C6 | YbC6 | SrC6 | KC8 | RbC8 | NaC3 | KC3 | LiC3 | NaC2 | LiC2 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tc (K) | 11.5 | 11.15 | 6.5 | 1.65 | 0.14 | 0.025 | 2.3-3.8 | 3.0 | <0.35 | 5.0 | 1.9 |
Regarding the origin of superconductivity in diamond, three alternative theories exist at the moment: conventional BCS theory based on phonon-mediated pairing, correlated impurity band theory[14] and spin-flip-driven pairing of holes weakly localized in the vicinity of the Fermi level.[15] Whereas there is no solid experimental support for either model, recent accurate measurements of isotopic shift of the transition temperature Tc upon boron and carbon isotopic substitutions favor the BCS theory.[16]
Silicon
It was suggested[1] that "Si and Ge, which also form in the diamond structure, may similarly exhibit superconductivity under the appropriate conditions", and indeed, discoveries of superconductivity in heavily boron doped Si (Si:B)[17] and SiC:B[18] have quickly followed. Similar to diamond, Si:B is type-II superconductor, but it has much smaller values of Tc = 0.4 K and Hc = 0.4 T. Superconductivity in Si:B was achieved by heavy doping (above 8 at.%), realized through a special non-equilibrium technique of gas immersion laser doping.
Silicon carbide
Superconductivity in SiC was achieved by heavy doping with boron[19] or aluminum.[20] Both the cubic (3C-SiC) and hexagonal (6H-SiC) phases are superconducting and show a very similar Tc of 1.5 K. A crucial difference is however observed for the magnetic field behavior between aluminum and boron doping: SiC:Al is type-II, same as Si:B. On the contrary, SiC:B is type-I. In attempt to explain this difference, it was noted that Si sites are more important than carbon sites for superconductivity in SiC. Whereas boron substitutes carbon in SiC, Al substitutes Si sites. Therefore, Al and B "see" different environment that might explain different properties of SiC:Al and SiC:B.[21]
Hydrogen Sulfide
At pressures above 90 GPa (gigapascal), hydrogen sulfide becomes a metallic conductor of electricity. When cooled below a critical temperature this high-pressure phase exhibits superconductivity. The critical temperature increases with pressure, ranging from 23 K at 100 GPa to 150 K at 200 GPa.[22] If hydrogen sulfide is pressurized at higher temperatures, then cooled, the critical temperature reaches 203 K (−70 °C), the highest accepted superconducting critical temperature as of 2015. By substituting a small part of sulfur with phosphorus and using even higher pressures, it has been predicted that it may be possible to raise the critical temperature to above 0 °C (273 K) and achieve room-temperature superconductivity.[23]
See also
- Conventional superconductor
- List of superconductors
- High-temperature superconductivity
- Room temperature superconductor
- Superconductivity
- Superconductor classification
- Technological applications of superconductivity
- Timeline of low-temperature technology
- Type-I superconductor
- Type-II superconductor
- Unconventional superconductor
- Silicon
- Silicon carbide
- Synthetic diamond
References
- 1 2 E. A. Ekimov; V. A. Sidorov; E. D. Bauer; N. N. Mel'nik; N. J. Curro; J. D. Thompson; S. M. Stishov (2004). "Superconductivity in diamond". Nature. 428 (6982): 542–545. arXiv:cond-mat/0404156. Bibcode:2004Natur.428..542E. doi:10.1038/nature02449. PMID 15057827.
L. Boeri, J. Kortus and O. K. Andersen "Three-Dimensional MgB2-Type Superconductivity in Hole-Doped Diamond",
K.-W. Lee and W. E. Pickett "Superconductivity in Boron-Doped Diamond",
X. Blase, Ch. Adessi and D. Connetable "Role of the Dopant in the Superconductivity of Diamond",
E. Bustarret et al. "Dependence of the Superconducting Transition Temperature on the Doping Level in Single-Crystalline Diamond Films" - free download - ↑ Gurevich V L, Larkin A I and Firsov Yu A (1962). Sov. Phys. Solid State. 4: 185.
- ↑ M. L. Cohen (1964). "The Existence of a Superconducting State in Semiconductors". Rev. Mod. Phys. 36: 240–243. Bibcode:1964RvMP...36..240C. doi:10.1103/RevModPhys.36.240.
- ↑ R.A. Hein; et al. (1964). "Superconductivity in Germanium Telluride". Phys. Rev. Lett. 12 (12): 320–322. Bibcode:1964PhRvL..12..320H. doi:10.1103/PhysRevLett.12.320.
- ↑ L. Finegold (1964). "Germanium Telluride: Specific Heat and Superconductivity". Phys. Rev. Lett. 13 (7): 233–234. Bibcode:1964PhRvL..13..233F. doi:10.1103/PhysRevLett.13.233.
- ↑ B. Stritzker; H. Wuhl (1976). "Superconductivity of amorphous Germanium produced by ion implantation". Zeitschrift für Physik B. 24 (4): 367–370. Bibcode:1976ZPhyB..24..367S. doi:10.1007/BF01351526.
- ↑ Y. Takano; et al. (2007). "Superconducting properties of homoepitaxial CVD diamond". Diam. Relat. Mater. 16 (4–7): 911–914. Bibcode:2007DRM....16..911T. doi:10.1016/j.diamond.2007.01.027.
- ↑ Y. Takano (2006). "Overview". Sci. Technol. Adv. Mater. 7: S1. Bibcode:2006STAdM...7S...1T. doi:10.1016/j.stam.2006.06.003.
- ↑ Z.K. Tang; et al. (2001). "Superconductivity in 4 Angstrom Single-Walled Carbon Nanotubes". Science. 292 (5526): 2462–5. Bibcode:2001Sci...292.2462T. doi:10.1126/science.1060470. PMID 11431560.
- ↑ M. Kociak; et al. (2001). "Superconductivity in Ropes of Single-Walled Carbon Nanotubes". Physical Review Letters. 86 (11): 2416–2419. arXiv:cond-mat/0010220. Bibcode:2001PhRvL..86.2416K. doi:10.1103/PhysRevLett.86.2416.
- ↑ M. Bockrath (2006). "Carbon nanotubes: The weakest link". Nature Physics. 2 (3): 155–156. Bibcode:2006NatPh...2..155B. doi:10.1038/nphys252.
- ↑ N. Emery; et al. (2008). "Synthesis and superconducting properties of CaC6". Sci. Technol. Adv. Mater. 9 (4): 044102. Bibcode:2008STAdM...9d4102E. doi:10.1088/1468-6996/9/4/044102. PMC 5099629. PMID 27878015.
- ↑ I.T Belash; et al. (1990). "Superconductivity of GIC with Li, Na and K". Synthetic Metals. 34: 455–460. doi:10.1016/0379-6779(89)90424-4.
- ↑ G. Baskaran (2008). "Impurity band Mott insulators: a new route to high Tc superconductivity". Sci. Technol. Adv. Mater. 9 (4): 044104. Bibcode:2008STAdM...9d4104B. doi:10.1088/1468-6996/9/4/044104. PMC 5099631. PMID 27878017.
- ↑ J. Mares; et al. (2008). "Selected topics related to the transport and superconductivity in boron-doped diamond". Sci. Technol. Adv. Mater. 9 (4): 044101. Bibcode:2008STAdM...9d4101M. doi:10.1088/1468-6996/9/4/044101. PMC 5099628. PMID 27878014.
- ↑ E. A. Ekimov; et al. (2008). "Structure and superconductivity of isotope-enriched boron-doped diamond". Sci. Technol. Adv. Mater. 9 (4): 044210. Bibcode:2008STAdM...9d4210E. doi:10.1088/1468-6996/9/4/044210. PMC 5099641. PMID 27878027.
- ↑ E. Bustarret; et al. (2006). "Superconductivity in doped cubic silicon". Nature. 444 (7118): 465–8. Bibcode:2006Natur.444..465B. doi:10.1038/nature05340. PMID 17122852.
- ↑ Zhi-An Ren; et al. (2007). "Superconductivity in Boron-doped SiC". J. Phys. Soc. Jpn. 76 (2): 103710. Bibcode:2007JPSJ...76b3710M. doi:10.1143/JPSJ.76.023710.
- ↑ M. Kriener; et al. (2008). "Superconductivity in heavily boron-doped silicon carbide". Sci. Technol. Adv. Mater. 9 (4): 044205. arXiv:0810.0056. Bibcode:2008STAdM...9d4205K. doi:10.1088/1468-6996/9/4/044205. PMC 5099636. PMID 27878022.
- ↑ T. Muranaka; et al. (2008). "Superconductivity in carrier-doped silicon carbide". Sci. Technol. Adv. Mater. 9 (4): 044204. Bibcode:2008STAdM...9d4204M. doi:10.1088/1468-6996/9/4/044204. PMC 5099635. PMID 27878021.
- ↑ Y. Yanase; N. Yorozu (2008). "Superconductivity in compensated and uncompensated semiconductors". Sci. Technol. Adv. Mater. 9 (4): 044201. Bibcode:2008STAdM...9d4201Y. doi:10.1088/1468-6996/9/4/044201. PMC 5099632. PMID 27878018.
- ↑ Drozdov, A.; Eremets, M. I.; Troyan, I. A. (2014). "Conventional superconductivity at 190 K at high pressures". arXiv:1412.0460 [cond-mat.supr-con].
- ↑ Cartlidge, Edwin (18 August 2015). "Superconductivity record sparks wave of follow-up physics". Nature News. Retrieved 18 August 2015.
External links
- International Workshop on superconductivity in Diamond and Related Materials 2005
- International Workshop on Superconductivity in Diamond and Related Materials 2008
- New Diamond and Frontier Carbon Technology Volume 17, No.1 Special Issue on Superconductivity in CVD Diamond
- Some papers on superconducting diamond