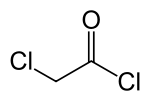
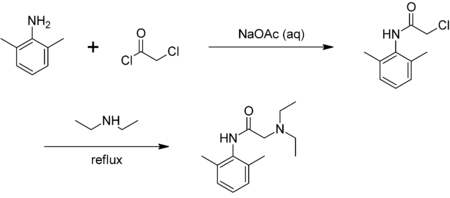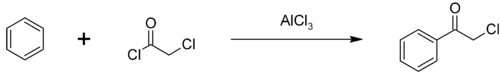Chloroacetyl chloride
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Chloroacetyl chloride | |
| Other names
2-Chloroacetyl chloride Chloroacetic acid chloride Chloroacetic chloride Monochloroacetyl chloride | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.001.065 |
| EC Number | 201-171-6 |
| KEGG | |
PubChem CID |
|
| |
| |
| Properties | |
| C2H2Cl2O | |
| Molar mass | 112.94 g·mol−1 |
| Appearance | Colorless to yellow liquid |
| Density | 1.42 g/mL |
| Melting point | −22 °C (−8 °F; 251 K) |
| Boiling point | 106 °C (223 °F; 379 K) |
| Reacts | |
| Vapor pressure | 19 mmHg (20°C)[1] |
| Hazards | |
| Safety data sheet | Oxford MSDS |
EU classification (DSD) (outdated) |
|
| Flash point | noncombustible [1] |
| US health exposure limits (NIOSH): | |
PEL (Permissible) |
none[1] |
REL (Recommended) |
TWA 0.05 ppm (0.2 mg/m3)[1] |
IDLH (Immediate danger) |
N.D.[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Chloroacetyl chloride is a chlorinated acyl chloride. It is a bifunctional compound, making it a useful building block chemical.
Production
Industrially, it is produced by the carbonylation of methylene chloride, oxidation of vinylidene chloride, or the addition of chlorine to ketene.[2] It may be prepared from chloroacetic acid and thionyl chloride, phosphorus pentachloride, or phosgene.
Reactions
Chloroacetyl chloride is bifunctional—the acyl chloride easily forms esters[3] and amides, while the other end of the molecule is able to form other linkages, e.g. with amines. The use of chloroacetyl chloride in the synthesis of lidocaine is illustrative:[4]
Applications
The major use of chloroacetyl chloride is as an intermediate in the production of herbicides in the chloroacetanilide family including metolachlor, acetochlor, alachlor and butachlor; an estimated 100 million pounds are used annually. Some chloroacetyl chloride is also used to produce phenacyl chloride, another chemical intermediate, also used as a tear gas.[2] Phenacyl chloride is synthesized in a Friedel-Crafts acylation of benzene, with an aluminium chloride catalyst:[5]
Safety
Like other acyl chlorides, reaction with other protic compounds such as amines, alcohols, and water generates hydrochloric acid, making it a lachrymator.
There is no regulated permissible exposure limit set by the Occupational Safety and Health Administration. However, the National Institute for Occupational Safety and Health has set a recommended exposure limit at 0.05 ppm over an eight-hour work day.[6]
References
- 1 2 3 4 5 "NIOSH Pocket Guide to Chemical Hazards #0120". National Institute for Occupational Safety and Health (NIOSH).
- 1 2 Paul R. Worsham (1993). "15. Halogenated Derivatives". In Zoeller, Joseph R.; Agreda, V. H. Acetic acid and its derivatives (Google Books excerpt). New York: M. Dekker. pp. 288–298. ISBN 0-8247-8792-7.
- ↑ Robert H. Baker and Frederick G. Bordwell (1955). "tert-Butyl acetate". Organic Syntheses. ; Collective Volume, 3
- ↑ T. J. Reilly (1999). "The Preparation of Lidocaine". J. Chem. Educ. 76 (11): 1557. doi:10.1021/ed076p1557.
- ↑ Nathan Levin and Walter H. Hartung (1955). "ω-Chloroisonitrosoacetophenone". Organic Syntheses. ; Collective Volume, 3, p. 191
- ↑ "NIOSH Pocket Guide to Chemical Hazards". Centers for Disease Control and Prevention. 2011.