Cage effect
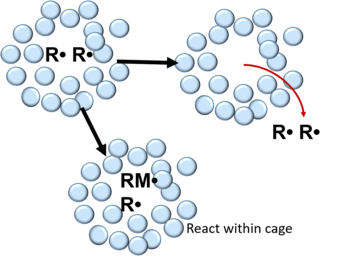
The cage effect in chemistry describes how the properties of a molecule are affected by its surroundings. First introduced by Franck and Rabinowitch [1][2] in 1934, the cage effect suggests that instead of acting as an individual particle, molecules in solvent are more accurately described as an encapsulated particle.[3][4] In order to interact with other molecules, the caged particle must diffuse from its solvent cage. The typical lifetime of a solvent cage is 10−11s.[5]
In free radical polymerization, radicals formed from the decomposition of an initiator molecule are surrounded by a cage consisting of solvent and/or monomer molecules.[4] Within the cage, the free radicals undergo many collisions leading to their recombination or mutual deactivation.[3][4][6] This can be described in the follow reaction:
After recombination, free radicals can either react with monomer molecules within the cage walls or diffuse out of the cage. In polymers, the probability of a free radical pair to escape recombination in the cage is 0.1 - 0.01 and 0.3-0.8 in liquids.[3]
Cage recombination efficiency
The cage effect can be quantitatively described as the cage recombination efficiency Fc where:
Here Fc is defined as the ratio of the rate constant for cage recombination (kc) to the sum of the rate constants for all cage processes.[6] According to mathematical models, Fc is dependent on changes on several parameters including radical size, shape, and solvent viscosity.[6][7][8] It is reported that the cage effect will increase with an increase in radical size and a decrease in radical mass.
Initiator efficiency
In free radical polymerization, the rate of initiation is dependent on how effective the initiator is.[4] Low initiator efficiency, ƒ, is largely attributed to the cage effect. The rate of initiation is described as:
where Ri is the rate of initiation, kd is the rate constant for initiator dissociation, [I] is the initial concentration of initiator. Initiator efficiency represents the fraction of primary radicals R·, that actually contribute to chain initiation. Due to the cage effect, free radicals can undergo mutual deactivation which produces stable products instead of initiating propagation - reducing the value of ƒ.[4]
References
- ↑ Rabinowitch, Franck (1934). "Some remarks about free radicals and the photochemisty of solutions". Transactions of the Faraday Society. 30: 120–130. doi:10.1039/tf9343000120 – via Scopus.
- ↑ Rabinowitch, E (1936). "The collison [sic] mechanism and the primary photochemical process in solutions". Transactions of the Faraday Society. 32: 1381–1387. doi:10.1039/tf9363201381 – via Socpus.
- 1 2 3 Denisov, E.T. (1984). "Cage effects in a polymer matrix". Macromolecular Chemistry and Physics. 8: 63–78. doi:10.1002/macp.1984.020081984106 – via Wiley Online Library.
- 1 2 3 4 5 6 Chanda, Manas (2013). Introduction to Polymer Science and Chemistry: A problem solving approach. New York: CRC Press. pp. 291, 301–303.
- ↑ Herk, L.; Feld, M.; Szwarc, M. (1961). "Studies of "Cage" Reactions". J. Am. Chem. Soc. 83: 2998–3005. doi:10.1021/ja01475a005.
- 1 2 3 4 5 Braden, Dale, A. (2001). "Solvent cage effects. I. Effect of radical mass and size on radical cage pair recombination efficiency. II. Is geminate recombination of polar radicals sensitive to solvent polarity?". Coordination Chemistry Reviews. 211: 279–294. doi:10.1016/s0010-8545(00)00287-3 – via Elsevier Science Direct.
- ↑ Noyes, R.M. (1954). "A Treatment of Chemical Kinetics with Special Applicability to Diffusion Controlled Reactions". J. Chem. Phys. 22: 1349. Bibcode:1954JChPh..22.1349N. doi:10.1063/1.1740394.
- ↑ Noyes, R.M. (1961). Progr. React. Kinet. 1: 129. Missing or empty
|title=(help)