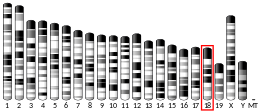
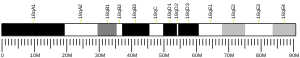
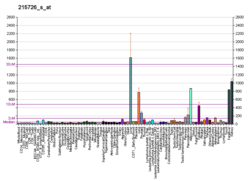
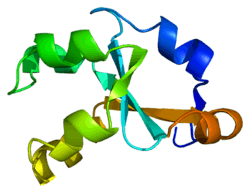Cytochrome b5, type A
Cytochrome b5, form A (gene name CYB5A), is a human microsomal cytochrome b5.[5]
Cytochrome b5 is a membrane bound hemoprotein which functions as an electron carrier for several membrane bound oxygenases. It has two isoforms produced by alternative splicing. Isoform 1 is bound to the cytoplasmic side of the endoplasmic reticulum. It has a C-terminal transmembrane alpha-helix. Isoform 2 was found in cytoplasm. Defects in CYB5A are the cause of type IV hereditary methemoglobinemia.
References
Further reading
- Ng S, Smith MB, Smith HT, Millett F (1977). "Effect of modification of individual cytochrome c lysines on the reaction with cytochrome b5". Biochemistry. 16 (23): 4975–8. doi:10.1021/bi00642a006. PMID 199233.
- Dailey HA, Strittmatter P (1979). "Modification and identification of cytochrome b5 carboxyl groups involved in protein-protein interaction with cytochrome b5 reductase". J. Biol. Chem. 254 (12): 5388–96. PMID 221468.
- Mitoma J, Ito A (1992). "The carboxy-terminal 10 amino acid residues of cytochrome b5 are necessary for its targeting to the endoplasmic reticulum". EMBO J. 11 (11): 4197–203. PMC 556930. PMID 1396600.
- Giordano SJ, Steggles AW (1991). "The human liver and reticulocyte cytochrome b5 mRNAs are products from a single gene". Biochem. Biophys. Res. Commun. 178 (1): 38–44. doi:10.1016/0006-291X(91)91776-9. PMID 1712589.
- Shephard EA, Povey S, Spurr NK, Phillips IR (1992). "Chromosomal localization of a cytochrome b5 gene to human chromosome 18 and a cytochrome b5 pseudogene to the X chromosome". Genomics. 11 (2): 302–8. doi:10.1016/0888-7543(91)90136-3. PMID 1840560.
- Strittmatter P, Hackett CS, Korza G, Ozols J (1991). "Characterization of the covalent cross-links of the active sites of amidinated cytochrome b5 and NADH:cytochrome b5 reductase". J. Biol. Chem. 265 (35): 21709–13. PMID 2123873.
- Ozols J (1989). "Structure of cytochrome b5 and its topology in the microsomal membrane". Biochim. Biophys. Acta. 997 (1–2): 121–30. doi:10.1016/0167-4838(89)90143-X. PMID 2752049.
- Yoo M, Steggles AW (1988). "The complete nucleotide sequence of human liver cytochrome b5 mRNA". Biochem. Biophys. Res. Commun. 156 (1): 576–80. doi:10.1016/S0006-291X(88)80881-7. PMID 3178851.
- Hegesh E, Hegesh J, Kaftory A (1986). "Congenital methemoglobinemia with a deficiency of cytochrome b5". N. Engl. J. Med. 314 (12): 757–61. doi:10.1056/NEJM198603203141206. PMID 3951505.
- Abe K, Kimura S, Kizawa R, et al. (1985). "Amino acid sequences of cytochrome b5 from human, porcine, and bovine erythrocytes and comparison with liver microsomal cytochrome b5". J. Biochem. 97 (6): 1659–68. PMID 4030743.
- Strittmatter P, Spatz L, Corcoran D, et al. (1975). "Purification and properties of rat liver microsomal stearyl coenzyme A desaturase". Proc. Natl. Acad. Sci. U.S.A. 71 (11): 4565–9. doi:10.1073/pnas.71.11.4565. PMC 433928. PMID 4373719.
- Rashid MA, Hagihara B, Kobayashi M, et al. (1974). "Structural studies of cytochrome b5. 3. Sequential studies on human liver cytochrome b5". J. Biochem. 74 (5): 985–1002. PMID 4770377.
- Nóbrega FG, Ozols J (1971). "Amino acid sequences of tryptic peptides of cytochromes b5 from microsomes of human, monkey, porcine, and chicken liver". J. Biol. Chem. 246 (6): 1706–17. PMID 4993957.
- Ozols J (1972). "Cytochrome b 5 from a normal human liver. Isolation and the partial amino acid sequence". J. Biol. Chem. 247 (7): 2242–5. PMID 5062820.
- Dailey HA, Strittmatter P (1980). "Characterization of the interaction of amphipathic cytochrome b5 with stearyl coenzyme A desaturase and NADPH:cytochrome P-450 reductase". J. Biol. Chem. 255 (11): 5184–9. PMID 6102994.
- De Silvestris M, D'Arrigo A, Borgese N (1995). "The targeting information of the mitochondrial outer membrane isoform of cytochrome b5 is contained within the carboxyl-terminal region". FEBS Lett. 370 (1–2): 69–74. doi:10.1016/0014-5793(95)00797-D. PMID 7649306.
- Li XR, Giordano SJ, Yoo M, Steggles AW (1995). "The isolation and characterization of the human cytochrome b5 gene". Biochem. Biophys. Res. Commun. 209 (3): 894–900. doi:10.1006/bbrc.1995.1582. PMID 7733981.
- Giordano SJ, Yoo M, Ward DC, et al. (1994). "The human cytochrome b5 gene and two of its pseudogenes are located on chromosomes 18q23, 14q31-32.1 and 20p11.2, respectively". Hum. Genet. 92 (6): 615–8. doi:10.1007/BF00420948. PMID 8262522.
- Guengerich FP, Johnson WW (1998). "Kinetics of ferric cytochrome P450 reduction by NADPH-cytochrome P450 reductase: rapid reduction in the absence of substrate and variations among cytochrome P450 systems". Biochemistry. 36 (48): 14741–50. doi:10.1021/bi9719399. PMID 9398194.
- Lee-Robichaud P, Akhtar ME, Akhtar M (1998). "Control of androgen biosynthesis in the human through the interaction of Arg347 and Arg358 of CYP17 with cytochrome b5". Biochem. J. 332. ( Pt 2): 293–6. PMC 1219480. PMID 9601054.
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.