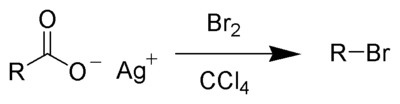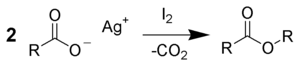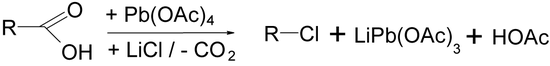Hunsdiecker reaction
| Hunsdiecker reaction | |
|---|---|
| Named after | Heinz Hunsdiecker Cläre Hunsdiecker Alexander Borodin |
| Reaction type | Substitution reaction |
| Identifiers | |
| Organic Chemistry Portal | hunsdiecker-reaction |
| RSC ontology ID | RXNO:0000106 |
The Hunsdiecker reaction (also called the Borodin reaction or the Hunsdiecker–Borodin reaction) is a name reaction in organic chemistry whereby silver salts of carboxylic acids react with a halogen to produce an organic halide.[1] It is an example of both a decarboxylation and a halogenation reaction as the product has one fewer carbon atoms than the starting material (lost as carbon dioxide) and a halogen atom is introduced its place. The reaction was first demonstrated by Alexander Borodin in his 1861 reports of the preparation of methyl bromide from silver acetate.[2][3] Shortly after, the approach was applied to the degradation of fatty acids in the laboratory of Adolf Lieben.[4][5] However, it is named for Cläre Hunsdiecker and her husband Heinz Hunsdiecker, whose work in the 1930s[6][7] developed it into a general method.[1] Several reviews have been published,[8][9] and a catalytic approach has been developed.[10]
History
Alexander Borodin first observed the reaction in 1861 when he prepared methyl bromide from silver acetate.[2][3] The reaction is a decarboxylation in that alkyl halide product has one fewer carbon atoms than its parent carboxylate, lost as carbon dioxide.[1][8]
Around the same time, Angelo Simonini was working as a student of Adolf Lieben at the University of Vienna, investigating the reactions of silver carboxylates with iodine.[8] They found that the products formed are determined by the stoichiometry within the reaction mixture. Using a carboxylate-to-iodine ratio of 1:1 leads to an alkyl iodide product, in line with Borodin's findings and the modern understanding of the Hunsdiecker reaction. However, a 2:1 ratio favours the formation of an ester product that arises from decarboxylation of one carboxylate and coupling the resulting alkyl chain with the other.[4][5]
Using a 3:2 ratio of reactants leads to the formation of a 1:1 mixture of both products.[4][5] These processes are sometimes known as the Simonini reaction rather than as modifications of the Hunsdiecker reaction.[8][9]
- 3 RCO
2Ag + 2 I
2 → RI + RCO
2R + 2 CO
2 + 3 AgI
It is now well established that mercuric oxide can also be used to effect this transformation.[11][12] The reaction has been applied to the preparation of ω-bromo esters with chain lengths between five and seventeen carbon atoms, with the preparation of methyl 5-bromovalerate published in Organic Syntheses as an exemplar.[13]
Reaction mechanism
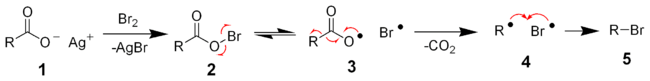
The reaction mechanism of the Hunsdiecker reaction is believed to involve organic radical intermediates. The silver salt of the carboxylic acid 1 will quickly react with bromine to form acyl hypohalite intermediate 2. Formation of the diradical pair 3 allows for radical decarboxylation to form the diradical pair 4, which will quickly recombine to form the desired organic halide 5. The trend in the yield of the resulting halide is primary > secondary > tertiary.[8][9]
Variations
Mercuric oxide
Lampman and Aumiller used mercuric oxide and bromine to prepare 1-bromo-3-chlorocyclobutane from 3-chlorocyclobutanecarboxylic acid in a modification of the Hunsdiecker reaction.[12] The product had previously been shown by Wiberg to react with molten sodium metal to form bicyclobutane via a Wurtz coupling in good yield.[14][15]
Kochi reaction
The Kochi reaction is a variation on the Hunsdiecker reaction developed by Jay Kochi that uses lead(IV) acetate and lithium chloride (lithium bromide can also be used) to effect the halogenation and decarboxylation.[16]
See also
References
- 1 2 3 Li, J. J. "Hunsdiecker–Borodin Reaction". Name Reactions: A Collection of Detailed Mechanisms and Synthetic Applications (5th ed.). Springer Science & Business Media. pp. 327–328. ISBN 9783319039794.
- 1 2 Borodin, A. (1861). "Ueber Bromvaleriansäure und Brombuttersäure" [About bromovaleric acid and bromobutyric acid]. Annalen der Chemie und Pharmacie (in German). 119: 121–123. doi:10.1002/jlac.18611190113.
- 1 2 Borodin, A. (1861). "Ueber de Monobrombaldriansäure und Monobrombuttersäure" [About the monobromovaleric acid and monobromobutyric acid]. Zeitschrift für Chemie und Pharmacie (in German). 4: 5–7.
- 1 2 3 Simonini, A. (1892). "Über den Abbau der fetten Säuren zu kohlenstoffärmeren Alkoholen" [About the breakdown of fatty acids to lower carbon alcohols]. Monatshefte für Chemie und verwandte Teile anderer Wissenschaften (in German). 13 (1): 320–325. doi:10.1007/BF01523646.
- 1 2 3 Simonini, A. (1893). "Über den Abbau der fetten Säuren zu kohlenstoffärmeren Alkoholen" [About the breakdown of fatty acids to lower carbon alcohols]. Monatshefte für Chemie und verwandte Teile anderer Wissenschaften (in German). 14 (1): 81–92. doi:10.1007/BF01517859.
- ↑ US patent 2176181, Hunsdiecker, C.; E. Vogt & H. Hunsdiecker, "Method of manufacturing organic chlorine and bromine derivatives", published 1939-10-17, assigned to Hunsdiecker, C.; Vogt, E.; Hunsdiecker, H.
- ↑ Hunsdiecker, H.; Hunsdiecker, C. (1942). "Über den Abbau der Salze aliphatischer Säuren durch Brom" [About the degradation of salts of aliphatic acids by bromine]. Chemische Berichte (in German). 75 (3): 291–297. doi:10.1002/cber.19420750309.
- 1 2 3 4 5 Johnson, R. G.; Ingham, R. K. (1956). "The Degradation of Carboxylic Acid Salts by Means of Halogen – the Hunsdiecker Reaction". Chem. Rev. 56 (2): 219–269. doi:10.1021/cr50008a002.
- 1 2 3 Wilson, C. V. (1957). "The Reaction of Halogens with Silver Salts of Carboxylic Acids". Org. React. 9: 332–387. doi:10.1002/0471264180.or009.05.
- ↑ Wang, Zhentao; Zhu, Lin; Yin, Feng; Su, Zhongquan; Li, Zhaodong; Li, Chaozhong (2012). "Silver-Catalyzed Decarboxylative Chlorination of Aliphatic Carboxylic Acids". Journal of the American Chemical Society. 134 (9): 4258–4263. doi:10.1021/ja210361z.
- ↑ Meek, J. S.; Osuga, D. T. (1963). "Bromocyclopropane". Org. Synth. 43: 9. doi:10.15227/orgsyn.043.0009. ; Coll. Vol., 5, p. 126
- 1 2 Lampman, G. M.; Aumiller, J. C. (1971). "Mercury(II) oxide-modified Hunsdiecker reaction: 1-Bromo-3-chlorocyclobutane". Org. Synth. 51: 106. doi:10.15227/orgsyn.051.0106. ; Coll. Vol., 6, p. 179
- ↑ Allen, C. F. H.; Wilson, C. V. (1946). "Methyl 5-bromovalerate (Valeric acid, δ-bromo-, methyl ester)". Org. Synth. 26: 52. doi:10.15227/orgsyn.026.0052. ; Coll. Vol., 3, p. 578
- ↑ Wiberg, K. B.; Lampman, G. M.; Ciula, R. P.; Connor, D. S.; Schertler, P.; Lavanish, J. (1965). "Bicyclo[1.1.0]butane". Tetrahedron. 21 (10): 2749–2769. doi:10.1016/S0040-4020(01)98361-9.
- ↑ Lampman, G. M.; Aumiller, J. C. (1971). "Bicyclo[1.1.0]butane". Org. Synth. 51: 55. doi:10.15227/orgsyn.051.0055. ; Coll. Vol., 6, p. 133
- ↑ Kochi, J. K. (1965). "A New Method for Halodecarboxylation of Acids Using Lead(IV) Acetate". Journal of the American Chemical Society. 87 (11): 2500–2502. doi:10.1021/ja01089a041.