Boranylium ions
A boranylium ion is an inorganic cation with the chemical formula BR+
2, where R represents a non-specific substituent. Being electron deficient, boranylium ions form adducts with Lewis bases. Boranylium ions have historical names that depend on the number of coordinated ligands:
- [BR
2]+
: borinium - [BR
2L]+
: borenium - [BR
2L
2]+
: boronium
Borenium ions are of some interest to academic research.[1][2][3][4][5]
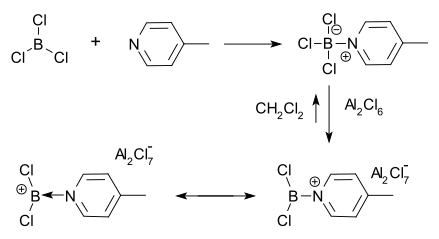
The first borenium ion was observed in 1970 by Ryschkewitsch & Wiggins.[6] They found that aluminum chloride dissolved in methylene chloride in the presence of the adduct 4-picoline.BCl3. A positive charge on boron was then inferred from proton NMR spectroscopy.
Related boron cations
Borinium ions have the formula [BX2]+,[7] where X− is usual a bulky amide (R2N−). Such species have linear geometry at boron and are coordinatively unsaturated. Boronium ions have the formula [L2BR2]+ (L = Lewis base). A well known example is (H3N)2BH2]+. Together with ammonia borane, this ion is produced by treating borane with ammonia. Boronium ions are tetrahedral and coordinatively saturated.[2][4]
Other non-classical boron cations are mononuclear boron di- and tri-cations with formula [L3BX]2+ and [L4B]3+, respectively.[8]
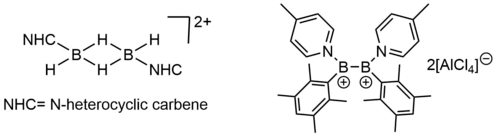
Other reported boron cations are dibora-dications (bis(borenium) dications), some examples are depicted below.[9][10]
References
- ↑ Ingleson, Michael J. (2015), "Fundamental and Applied Properties of Borocations", Synthesis and Application of Organoboron Compounds, Springer International Publishing, pp. 39–71, doi:10.1007/978-3-319-13054-5_2, ISBN 9783319130538
- 1 2 De Vries, Timothy S.; Prokofjevs, Aleksandrs; Vedejs, Edwin (2012-04-20). "Cationic Tricoordinate Boron Intermediates: Borenium Chemistry from the Organic Perspective". Chemical Reviews. 112 (7): 4246–4282. doi:10.1021/cr200133c. ISSN 0009-2665. PMC 3394883. PMID 22519545.
- ↑ P. Koelle and H. Nöth (1985). "The chemistry of borinium and borenium ions". Chemical Reviews. 85: 399–418. doi:10.1021/cr00069a004.
- 1 2 Piers, W. E., Bourke, S. C. and Conroy, K. D. (2005), Borinium, Borenium, and Boronium Ions: Synthesis, Reactivity, and Applications. Angew. Chem. Int. Ed., 44: 5016–5036. doi:10.1002/anie.200500402
- ↑ Eisenberger, P.; Crudden, C. M. (2017). "Borocation catalysis". Dalton Transactions. 46 (15): 4874–4887. doi:10.1039/c6dt04232e. ISSN 1477-9226.
- ↑ George E. Ryschkewitsch and J. W. Wiggins (1970). "Trigonal boron cation". Journal of the American Chemical Society. 92: 1790–1791. doi:10.1021/ja00709a079.
- ↑ Shoji, Yoshiaki; Tanaka, Naoki; Mikami, Koichiro; Uchiyama, Masanobu; Fukushima, Takanori (2014-05-11). "A two-coordinate boron cation featuring C–B+–C bonding". Nature Chemistry. 6 (6): 498–503. doi:10.1038/nchem.1948. ISSN 1755-4330.
- ↑ Vargas-Baca, Ignacio; Findlater, Michael; Powell, Adam; Vasudevan, Kalyan V.; Cowley, Alan H. (2008). "Boron di- and tri-cations". Dalton Transactions. 0 (45): 6421. doi:10.1039/b810575h. ISSN 1477-9226.
- ↑ Prokofjevs, Aleksandrs; Kampf, Jeff W.; Solovyev, Andrey; Curran, Dennis P.; Vedejs, Edwin (2013-10-10). "Weakly Stabilized Primary Borenium Cations and Their Dicationic Dimers". Journal of the American Chemical Society. 135 (42): 15686–15689. doi:10.1021/ja407458k. ISSN 0002-7863. PMC 3857331. PMID 24087933.
- ↑ Arnold, Nicole; Braunschweig, Holger; Dewhurst, Rian D.; Hupp, Florian; Radacki, Krzysztof; Trumpp, Alexandra (2016-08-12). "Desymmetrizing Electron-Deficient Diboranes(4): Diverse Products and Their Reactivity". Chemistry - A European Journal. 22 (39): 13927–13934. doi:10.1002/chem.201602805. ISSN 0947-6539.