AMP deaminase 2
AMP deaminase 2 is an enzyme that in humans is encoded by the AMPD2 gene.[5][6]
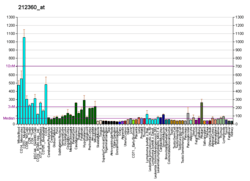
High AMPD2 expression levels correlate with poor patient outcome and a proliferative tumor phenotype in undifferentiated pleomorphic sarcoma (UPS). [7]
References
- 1 2 3 GRCh38: Ensembl release 89: ENSG00000116337 - Ensembl, May 2017
- 1 2 3 GRCm38: Ensembl release 89: ENSMUSG00000027889 - Ensembl, May 2017
- ↑ "Human PubMed Reference:".
- ↑ "Mouse PubMed Reference:".
- ↑ Mahnke-Zizelman DK, Sabina RL (Nov 1992). "Cloning of human AMP deaminase isoform E cDNAs. Evidence for a third AMPD gene exhibiting alternatively spliced 5'-exons". J Biol Chem. 267 (29): 20866–77. PMID 1400401.
- ↑ "Entrez Gene: AMPD2 adenosine monophosphate deaminase 2 (isoform L)".
- ↑ Orth MF, Gerke JS, Knösel T, Altendorf-Hofmann A, Musa J, Alba-Rubio R, et al. (September 2018). "Functional genomics identifies AMPD2 as a new prognostic marker for undifferentiated pleomorphic sarcoma". International Journal of Cancer. doi:10.1002/ijc.31903. PMID 30267407.
External links
- Human AMPD2 genome location and AMPD2 gene details page in the UCSC Genome Browser.
Further reading
- Bausch-Jurken MT, Mahnke-Zizelman DK, Morisaki T, Sabina RL (1992). "Molecular cloning of AMP deaminase isoform L. Sequence and bacterial expression of human AMPD2 cDNA". J. Biol. Chem. 267 (31): 22407–13. PMID 1429593.
- Van den Bergh F, Sabina RL (1996). "Characterization of human AMP deaminase 2 (AMPD2) gene expression reveals alternative transcripts encoding variable N-terminal extensions of isoform L". Biochem. J. 312 (Pt 2): 401–10. doi:10.1042/bj3120401. PMC 1136276. PMID 8526848.
- Mahnke-Zizelman DK, van den Bergh F, Bausch-Jurken MT, et al. (1996). "Cloning, sequence and characterization of the human AMPD2 gene: evidence for transcriptional regulation by two closely spaced promoters". Biochim. Biophys. Acta. 1308 (2): 122–32. doi:10.1016/0167-4781(96)00089-9. PMID 8764830.
- Haas AL, Sabina RL (2003). "N-terminal extensions of the human AMPD2 polypeptide influence ATP regulation of isoform L". Biochem. Biophys. Res. Commun. 305 (2): 421–7. doi:10.1016/S0006-291X(03)00787-3. PMID 12745092.
- Beausoleil SA, Villén J, Gerber SA, et al. (2006). "A probability-based approach for high-throughput protein phosphorylation analysis and site localization". Nat. Biotechnol. 24 (10): 1285–92. doi:10.1038/nbt1240. PMID 16964243.
- Olsen JV, Blagoev B, Gnad F, et al. (2006). "Global, in vivo, and site-specific phosphorylation dynamics in signaling networks". Cell. 127 (3): 635–48. doi:10.1016/j.cell.2006.09.026. PMID 17081983.
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.