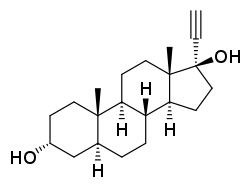
Ethynylandrostanediol
 | |
| Clinical data | |
|---|---|
| Synonyms | HE3235; Apoptone; 17α-Ethynyl-5α-androstane-3α,17β-diol; 5α-Pregn-20-yne-3α,17α-diol |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C21H32O2 |
| Molar mass | 316.49 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Ethynylandrostanediol (developmental code name HE-3235), or 17α-ethynyl-5α-androstane-3α,17β-diol, also known as Apoptone, is a synthetic, orally active, 17α-substituted analogue of the androstanediols which was under development for the treatment of prostate cancer and breast cancer but was discontinued.[1][2][3] It has very low affinity for steroid receptors, including the AR, ERα, ERβ, PR, and GR, and its mechanism of action is not well-characterized.[1][4] It produces 5α-dihydroethisterone as an active metabolite, which may contribute importantly to its biological activity.[1]
References
- 1 2 3 Ahlem C, Kennedy M, Page T, Bell D, Delorme E, Villegas S, Reading C, White S, Stickney D, Frincke J (2012). "17α-alkynyl 3α, 17β-androstanediol non-clinical and clinical pharmacology, pharmacokinetics and metabolism". Invest New Drugs. 30 (1): 59–78. doi:10.1007/s10637-010-9517-0. PMID 20814732.
- ↑ Trauger R, Corey E, Bell D, White S, Garsd A, Stickney D, Reading C, Frincke J (2009). "Inhibition of androstenediol-dependent LNCaP tumour growth by 17alpha-ethynyl-5alpha-androstane-3alpha, 17beta-diol (HE3235)". Br. J. Cancer. 100 (7): 1068–72. doi:10.1038/sj.bjc.6604987. PMC 2669987. PMID 19337256.
- ↑ http://webcache.googleusercontent.com/search?q=cache:Jy1DYIHEUtQJ:adisinsight.springer.com/drugs/800025305
- ↑ Koreckij TD, Trauger RJ, Montgomery RB, Pitts TE, Coleman I, Nguyen H, Reading CL, Nelson PS, Vessella RL, Corey E (2009). "HE3235 inhibits growth of castration-resistant prostate cancer". Neoplasia. 11 (11): 1216–25. PMC 2767223. PMID 19881957.
External links
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.