1-Tetralone
 | |
 | |
| Names | |
|---|---|
| IUPAC name
3,4-Dihydro-2H-naphthalen-1-one | |
| Other names
α-Tetralone; 1-Tetralone | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.007.692 |
PubChem CID |
|
| |
| |
| Properties | |
| C10H10O | |
| Molar mass | 146.19 g·mol−1 |
| Appearance | clear light yellow[2] to dark brown[3] liquid |
| Density | * 1.099 g·cm−3 (25 °C)[3] |
| Melting point | 2–7 °C[3] |
| Boiling point | * 255–257 °C[4]
|
| insoluble[2] | |
| Solubility | soluble in diethylether,[5] benzene,[6] toluene und xylene[7] |
| Vapor pressure | 2.7 Pa (20 °C)[2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
1-Tetralone is a bicyclic aromatic hydrocarbon with an α-keto group (a benzocycloalkanone) and can also be regarded as benzo-fused cyclohexanone. It is used as starting material for agricultural and pharmaceutical agents.
Preparation
The carbon skeleton of 1-tetralone is also found in natural products such as the so-called Aristelegone A (4,7-dimethyl-6-methoxy-1-tetralone) from the family of Aristolochiaceae used in traditional Chinese medicine.[8]
1-Tetralone via oxidation of 1,2,3,4-tetrahydronaphthalene
As already described in 1933 by Heinrich Hock, 1,2,3,4-tetrahydronaphthalene tends to autoxidize and gradually forms the 1-hydroperoxide with atmospheric oxygen.[9] The heavy metal ion catalyzed air oxidation of 1,2,3,4-tetrahydronaphthalene with Cr3+[10] or Cu2+ in the liquid phase leads via the hydroperoxide to a mixture of the intermediate 1-tetralol and the final product 1-tetralone.[11]

The boiling points of the main component 1-tetralone (255-257 °C) and the minor component 1-tetralol (255 °C)[4] are virtually identical, the latter is therefore removed by a chemical reaction.[6]
1-Tetralone by Friedel-Crafts reaction of 4-phenylbutyric acid
The starting compound 4-phenylbutanoic acid (whose sodium salt sodium phenylbutyrate is used to treat hyperammonaemia) is accessible from 3-benzoylpropanoic acid via catalytic hydrogenation, using a palladium contact catalyst with a yield of 96%.[5] 3-Benzoylpropanoic acid[12] itself can be obtained by a Haworth reaction (a variant of the Friedel-Crafts reaction) from benzene and succinic anhydride in 77-82 % yield. A recent patent claims the synthesis of 4-phenylbutanoic acid via a Friedel-Crafts acylation of benzene and γ-butyrolactone with aluminum chloride at 60 °C followed by a work up with dilute sodium hydroxide solution and subsequent acidification in 94% crude yield and 81% pure yield.[13]
The intramolecular cyclization of 4-phenylbutanoic acid to 1-tetralone can be achieved by heating with polyphosphoric acid in 75-86% yield.[5]

The acid-catalyzed cyclization can also be carried out with methanesulfonic acid.[14] The reaction yield is between 23 and 80%, it has been described as a teaching experiment for chemistry lessons.[15] 4-Phenylbutanoic acid can also be quantitatively converted into 1-tetralone by heating to 180 °C and addition of catalytic amounts of strong Lewis acids such as bismuth(III)bis(trifluoromethanesulfonyl)amide[16] [Bi(NTf2)3], which is relatively easily accessible.[7]
1-Tetralone by Friedel-Crafts reaction of 4-phenylbutanoic acid chloride
The use of the acid chloride (by reaction with phosphorus pentachloride) with overstoichiometric amounts of tin(IV) chloride (SnCl4) allows significantly shorter reaction times than the Friedel-Crafts acylation with 4-phenylbutanoic acid, total yields of 85-91 % be achieved according to the reported "method B".[6]

4-Phenylbutanoic acid chlorides with electron-donating groups can be cyclized to 1-tetralones under mild reaction conditions in yields greater than 90% using the strong hydrogen-bonding solvent hexafluoroisopropanol (HFIP).[17]
1-Tetralone by Friedel-Crafts reaction of γ-butyrolactone
The acylation of benzene with γ-butyrolactone with excess aluminum chloride produces 1-tetralone in yields of 91-96%.[6]

A disadvantage of many variants of the Friedel-Crafts acylation is the use of large amounts of AlCl3, polyphosphoric acid or PCl5 for the preparation of the used acid chlorides, which give rise to disproportionate difficulties and produce major volumes of waste.
For the reaction of benzene with γ-butyrolactone, the use of solid acidic catalysts based on zeolites and aluminosilicates was proposed, but no statement of their efficiency was given.[18] However, the seven-membered ketone 1-benzosuberone is accessible using acidic catalysts, from the six-membered lactone δ-valerolactone.

Properties
1-Tetralone is a clear, light yellow to dark brown liquid with a faint odor,[19] which is immiscible with water. It is miscible with non-polar organic solvents.
Toxicological studies were dermally performed with rabbits, with an LD50 of 2192 mg·kg−1 body weight being observed.[3]
The refractive index measured was 1.5672 (20 °C, 589 nm)[4] to 1.5695.[20]
Use
1-Tetralone can be reduced via a Birch reduction with lithium in liquid ammonia to 1,2,3,4-tetrahydronaphthalene in a with 96% yield.[21] The keto group can also be reduced to a secondary alcohol giving 1-tetralol in 70% yield, when a modified process is applied, using the addition of aqueous ammonium chloride solution after evaporation of the ammonia.[22]

With calcium in liquid ammonia, 1-tetralone is reduced to 1-tetralol at -33 °C in 81% yield.[23]
The methylene group in α-position to the keto group is particularly reactive and can be converted with formaldehyde (in the form of the trimeric paraldehyde) to 2-methylene-1-tetralone in the presence of the trifluoroacetic acid salt of N-methylaniline with yields up to 91% .
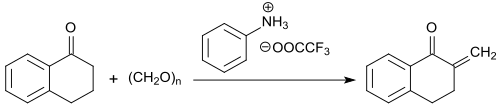
The 2-methylene ketone is stable at temperatures below -5 °C, but fully polymerizes at room temperature within 12 hours.[24]
In the Pfitzinger reaction of 1-tetralone with isatin, a compound called tetrofan (3,4-dihydro-1,2-benzacridine-5-carboxylic acid) is formed.

The reactivity of the α-methylene group is also exploited in the reaction of 1-tetralone with methanol at 270-290 °C, which produces via dehydrogenation and formation of the aromatic naphthalene ring system 2-methyl-1-naphthol in 66% yield.[25]

In the reaction of the oxime of 1-tetralone with acetic anhydride, aromatization of the cycloalkanone ring gives N-(1-naphthyl)acetamide[26] which acts like 2-(1-Naphthyl)acetic acid as a synthetic auxin.
acetamid.svg.png)
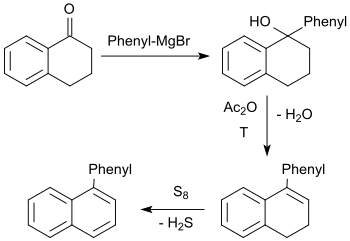
The tertiary alcohol formed in the Grignard reaction of 1-tetralone with phenylmagnesium bromide reacts with acetic anhydride upon elimination of water to 1-phenyl-3,4-dihydronaphthalene, which is dehydrated with elemental sulfur in an overall yield of about 45% to 1-phenylnaphthalene.[27]
The ruthenium(II)-catalyzed arylation of 1-tetralone using phenyl boronic acid neopentyl glycol ester gives 8-phenyl-1-tetralone in up to 86% yield.[28]

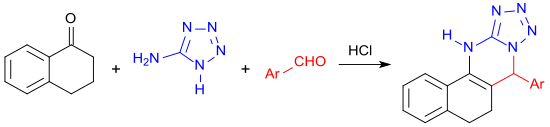
With 5-aminotetrazole and an aromatic aldehyde, 1-tetralone reacts in a multi-component reaction under microwave irradiation to form a four-membered heterocyclic ring system.[29]
The by far the most important application of 1-tetralone is in the synthesis of 1-naphthol by aromatizing dehydrogenation, e.g. on platinum catalysts at temperatures of 200 to 450 °C.[30]
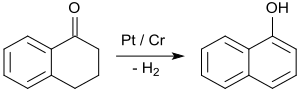
1-Naphthol is the starting material for the insecticide carbaryl and the beta-blockers propranolol[31] and nadolol,[32][33] as well as for the antidepressant sertraline[34] and the anti-protozoan therapeutic atovaquone.[35]
The use of 1-tetralone as a poison against clothes moths[19] has not prevailed despite its "hardly by the human sense noticeable unpleasant odor" on the market.
References
- ↑ α-Tetralone at Sigma-Aldrich
- 1 2 3 "alpha-Tetralone 529-34-0 | TCI Deutschland GmbH". www.tcichemicals.com (in German). Retrieved 2017-12-17.
- 1 2 3 4 5 Sigma-Aldrich Co., α-Tetralon. Retrieved on 25. November 2017.
- 1 2 3 William M. Haynes (2016), CRC Handbook of Chemistry and Physics, 97th Edition, Boca Raton, FL, U.S.A.: CRC Press, pp. 3–504, ISBN 978-1-4987-5429-3
- 1 2 3 "α-Tetralone". Organic Syntheses. doi:10.15227/orgsyn.020.0094.
- 1 2 3 4 "α-Tetralone". Organic Syntheses. doi:10.15227/orgsyn.035.0095.
- 1 2 D.-M. Cui, M. Kawamura, S. Shimada, T. Hayashi, M. Tanaka (2003), "Synthesis of 1-tetralones by intramolecular Friedel-Crafts reaction of 4-arylbutyric acids using Lewis acid catalysts" (in German), Tetrahedron Lett. 44 (21): pp. 4007–4010, doi:10.1016/S0040-4039(03)00855-4
- ↑ P.-C. Kuo, Y.-C. Li, T.-S. Wu (2012), "Chemical constituents and pharmacology of the Aristolochia species" (in German), eJTCM 2 (4): pp. 249–266, doi:10.1016/S2225-4110(16)30111-0
- ↑ H. Hock, W. Susemihl (1933), "Autoxydation von Kohlenwasserstoffen: Über ein durch Autoxydation erhaltenes Tetrahydro-naphthalin-peroxyd (I. Mitteil.)" (in German), Ber. Dtsch. Chem. Ges. 66 (1): pp. 61–68, doi:10.1002/cber.19330660113
- ↑ S. Bhattacharjee, Y.-R. Lee, W.-S. Ahn (2017), "Oxidation of tetraline to 1-tetralone over CrAPO-5" (in German), Korean J. Chem. Eng. 34 (3): pp. 701–705, doi:10.1007/s11814-016-0310-4
- ↑ US 4473711, R.W. Coon, "Liquid-phase process for oxidation of tetralin"
- ↑ "β-Benzoylpropionic acid". Organic Syntheses. doi:10.15227/orgsyn.013.0012.
- ↑ US 6372938, S.R. Burzynski, L. Musial, "Synthesis of 4-Phenylbutyric acid"
- ↑ V. Premasagar, V.A. Palaniswamy, E.J. Eisenbraun (1981), "Methanesulfonic acid catalyzed cyclization of 3-arylpropanoic and 4-arylbutanoic acids to 1-indanones and 1-tetralones" (in German), J. Org. Chem. 46 (14): pp. 2974–2976, doi:10.1021/jo00325a028
- ↑ M.S. Holden, R.D. Crouch, K.A. Barker (2005), "Formation of α-tetralone by intramolecular Friedel-Crafts acylation" (in German), J. Chem. Educ. 82 (6): pp. 934–935, doi:10.1021/ed082p934
- ↑ S. Antoniotti, E. Dunach (2008), "Facile preparation of metallic triflates and triflimidates by oxidative dissolution of metal powders" (in German), Chem. Commun. 8: pp. 993–995, doi:10.1039/B717689A
- ↑ H. Motiwala, R.H. Vekariya, J. Aubé (2015), "Intramolecular Friedel-Crafts acylation reaction promoted by 1,1,1,3,3,3-hexafluoro-2-propanol" (in German), Org. Lett. 17: pp. 5484–5487, doi:10.1021/acs.orglett.5b02851
- ↑ "SDK develops new catalyst for tetralone synthesis". Showa Denko K.K. News Release 2004 (in German). Showa Denko K.K. Retrieved 2017-12-01.
- 1 2 DE 357063, "Verfahren, um Pelzwerk und Wollstoffe gegen Motten und andere Insekten zu schützen"
- ↑ "Data sheet 1-Tetralone, 97% at AlfaAesar".
- ↑ S.S. Hall, S.D. Lipsky, F.J. McEnroe, A.P. Bartels (1971), "Lithium-ammonia reduction of aromatic ketones to aromatic hydrocarbons" (in German), J. Org. Chem. 38 (18): pp. 2588–2591, doi:10.1021/jo00817a004
- ↑ Z. Marcinow, P.W. Rabideau (1988), "Metal-ammonia reduction of α-tetralone. Competition between ring reduction, carbonyl reduction, and dimer formation" (in German), J. Org. Chem. 53 (9): pp. 2117–2119, doi:10.1021/jo00244a054
- ↑ J.R. Hwu, Y.S. Wein, Y.-J. Leu (1996), "Calcium metal in liquid ammonia for selective reduction of organic compounds" (in German), J. Org. Chem. 61 (4): pp. 1493–1499, doi:10.1021/jo951219c
- ↑ "Methylene ketones and aldehydes by simple, direct methylene transfer: 2-Methylene-1-oxo-1,2,3,4-tetrahydronaphthalene". Organic Syntheses. doi:10.15227/orgsyn.060.0088.
- ↑ I. Yuranov, L. Kiwi-Minsker, A. Renken (2002), "One-step vapour-phase synthesis of 2-methyl-1-naphthol from 1-tetralone" (in German), Appl. Catal. A 226 (1–2): pp. 193–198, doi:10.1016/S0926-860X(01)00902-4
- ↑ M.S. Newman, W.M. Hung (1973), "An improved aromatization of α-tetralone oximes to N-(1-naphthyl)acetamides" (in German), J. Org. Chem. 38 (23): pp. 4073–4074, doi:10.1021/jo00987a029
- ↑ "1-Phenylnaphthalene". Organic Syntheses. doi:10.15227/orgsyn.024.0084.
- ↑ "Ruthenium-catalyzed arylation of ortho C-H bond in an aromatic with an arylboronate: 8-Phenyl-1-tetralone". Organic Syntheses. doi:10.15227/orgsyn.087.0209.
- ↑ G.P. Kantin, M. Krasavin (2016), "Reaction of α-tetralone, 1H-tetrazol-5-amine, and aromatic aldehydes upon microwave irradiation – a convenient method for the synthesis of 5,6,7,12-tetrahydrobenzo[h]tetrazolo[5,1-b]quinazolines" (in German), Chem. Heterocycl. Compd. 52 (11): pp. 918–922, doi:10.1007/s10593-017-1985-0
- ↑ DE 2421745, K. Kudo, T. Ohmae, A. Uno, "Verfahren zur Herstellung von α-Naphthol durch katalytische Dehydrierung von α-Tetralon"
- ↑ C. Kaiser, T. Jen, E. Garvey, W.D. Bowen, D.F. Colella, J.R. Wardell Jr. (1977), "Adrenergic agents. 4. Substituted phenoxypropanolamine derivatives as potential β-adrenergic agonists" (in German), J. Med. Chem. 20 (5): pp. 687–689, doi:10.1021/jm00215a014
- ↑ M.E. Condon et al. (1978), "Nondepressant β-adrenergic blocking agents. 1. Substituted 3-amino-1-(5,6,7,8-tetrahydro-1-naphthoxy)-2-propanols" (in German), J. Med. Chem. 21 (9): pp. 913–922, doi:10.1021/jm00207a014
- ↑ DE 2258995, F.R. Hauck, C.M. Cimarusti, V.L. Narayan, "2,3-cis-1,2,3,4-Tetrahydro-5[2-hydroxy-3-(tert.-butylamino)-propoxy]-2,3-naphthalindiol"
- ↑ K. Vukics, T. Fodor, J. Fischer, I. Fellevári, S. Lévai (2002), "Improved industrial synthesis of antidepressant Sertraline" (in German), Org. Proc. Res. Dev. 6 (1): pp. 82–85, doi:10.1021/op0100549
- ↑ B.N. Roy, G.P. Singh, P.S. Lathi, M.K. Agarwal (2013), "A novel process for synthesis of Atovaquone" (in German), Indian J. Chem. 52B: pp. 1299–1312, http://nopr.niscair.res.in/bitstream/123456789/21838/1/IJCB%2052B%2810%29%201299-1312.pdf