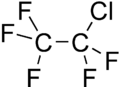
Chloropentafluoroethane
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
1-chloro-1,1,2,2,2-pentafluoroethane | |||
| Other names
Freon 115, CFC-115, R-115, Fluorocarbon-115, Genetron 115, Halocarbon 115, Monochloropentafluoroethane | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.854 | ||
| EC Number | 200-938-2 | ||
| E number | E945 (glazing agents, ...) | ||
PubChem CID |
|||
| RTECS number | KH7877500 | ||
| UNII | |||
| UN number | 1020 | ||
| |||
| |||
| Properties | |||
| C2ClF5 | |||
| Molar mass | 154.466 g/mol | ||
| Appearance | Colorless gas | ||
| Odor | Ethereal | ||
| Melting point | −99 °C (−146 °F; 174 K) | ||
| Boiling point | −39.1 °C (−38.4 °F; 234.1 K) | ||
| 59 mg/L | |||
| Vapor pressure | 7.9 atm (21°C)[1] | ||
| Hazards | |||
| Main hazards | In high concentrations may cause asphyxiation.[2] | ||
| GHS pictograms |   | ||
| GHS signal word | Warning | ||
| H280, H420 | |||
| P410+403, P502 | |||
| Flash point | 70.4 °C (158.7 °F; 343.5 K) | ||
| US health exposure limits (NIOSH): | |||
PEL (Permissible) |
none[1] | ||
REL (Recommended) |
TWA 1000 ppm (6320 mg/m3)[1] | ||
IDLH (Immediate danger) |
N.D.[1] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Chloropentafluoroethane is a chlorofluorocarbon once used as a refrigerant. Its production and consumption has been banned since 1 January 1996 under the Montreal Protocol because of its ozone-depleting potential.[3]
References
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.