Introduction
The number of known organic compounds is quite large. In fact, there are many times more organic compounds known than all the other (inorganic) compounds discovered so far, about 7 million organic compounds in total. Fortunately, organic chemicals consist of a relatively few similar parts, combined in different ways, that allow us to predict how a compound we have never seen before may react, by comparing how other molecules containing the same types of parts are known to react.
These parts of organic molecules are called functional groups. The identification of functional groups and the ability to predict reactivity based on functional group properties is one of the cornerstones of organic chemistry.
Functional groups are specific atoms, ions, or groups of atoms having consistent properties. A functional group makes up part of a larger molecule.
For example, -OH, the hydroxyl group that characterizes alcohols, is an oxygen with a hydrogen attached. It could be found on any number of different molecules.
Just as elements have distinctive properties, functional groups have characteristic chemistries. An -OH group on one molecule will tend to react similarly, although perhaps not identically, to an -OH on another molecule.
Organic reactions usually take place at the functional group, so learning about the reactivities of functional groups will prepare you to understand many other things about organic chemistry.
Memorizing Functional Groups
Don't assume that you can simply skim over the functional groups and move on. As you proceed through the text, the writing will be in terms of functional groups. It will be assumed that the student is familiar with most of the ones in the tables below. It's simply impossible to discuss chemistry without knowing the "lingo". It's like trying to learn French without first learning the meaning of some of the words.
One of the easiest ways to learn functional groups is by making flash cards. Get a pack of index cards and write the name of the functional group on one side, and draw its chemical representation on the other.
For now, a list of the most important ones you should know is provided here. Your initial set of cards should include, at the very least: Alkene, Alkyne, Alkyl halide (or Haloalkane), Alcohol, Aldehyde, Ketone, Carboxylic Acid, Acyl Chloride (or Acid Chloride), Ester, Ether, Amine, Sulfide, and Thiol. After you've learned all these, add a couple more cards and learn those. Then add a few more and learn those. Every functional group below is eventually discussed at one point or another in the book. But the above list will give you what you need to continue on.
And don't just look at the cards. Say and write the names and draw the structures. To test yourself, try going through your cards and looking at the names and then drawing their structure on a sheet of paper. Then try going through and looking at the structures and naming them. Writing is a good technique to help you memorize, because it is more active than simply reading. Once you have the minimal list above memorized backwards and forwards, you're ready to move on. But don't stop learning the groups. If you choose to move on without learning the "lingo", then you're not going to understand the language of the chapters to come. Again, using the French analogy, it's like trying to ignore learning the vocabulary and then picking up a novel in French and expecting to be able to read it.
Functional groups containing ...
In organic chemistry functional groups are submolecular structural motifs, characterized by specific elemental composition and connectivity, that confer reactivity upon the molecule that contains them.
Common functional groups include:
| Chemical class | Group | Formula | Graphical Formula | Prefix | Suffix | Example |
|---|---|---|---|---|---|---|
| Acyl halide | Haloformyl | RCOX |  |
haloformyl- | -oyl halide | 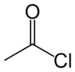 Acetyl chloride (Ethanoyl chloride) |
| Alcohol | Hydroxyl | OH | -skeletal.png) |
hydroxy- | -ol | 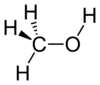 Methanol |
| Aldehyde | Aldehyde | RCHO |  |
oxo- | -al |  :Acetaldehyde (Ethanal) |
| Alkane | Alkyl | RHn | alkyl- | -ane | 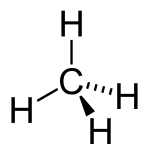 Methane | |
| Alkene | Alkenyl | R2C=CR2 | 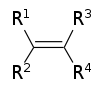 |
alkenyl- | -ene |  Ethylene (Ethene) |
| Alkyne | Alkynyl | RC≡CR' | alkynyl- | -yne | Acetylene (Ethyne) | |
| Amide | Carboxamide | RCONR2 | -skeletal.png) |
carboxamido- | -amide | 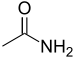 Acetamide (Ethanamide) |
| Amines | Primary amine | RNH2 | amino- | -amine |  Methylamine (Methanamine) | |
| Secondary amine | R2NH | .png) |
amino- | -amine | Dimethylamine | |
| Tertiary amine | R3N | .png) |
amino- | -amine |  Trimethylamine | |
| 4° ammonium ion | R4N+ |  |
ammonio- | -ammonium | Choline | |
| Azo compound | Azo (Diimide) |
RN2R' |  |
azo- | -diazene |  Methyl orange |
| Toluene derivative | Benzyl | RCH2C6H5 RBn |
benzyl- | 1-(substituent)toluene | Benzyl bromide (1-Bromotoluene) | |
| Carbonate | Carbonate ester | ROCOOR | alkyl carbonate | |||
| Carboxylate | Carboxylate | RCOO− | 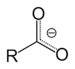
|
carboxy- | -oate | Sodium acetate (Sodium ethanoate) |
| Carboxylic acid | Carboxyl | RCOOH | 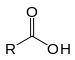 |
carboxy- | -oic acid | 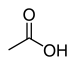 Acetic acid (Ethanoic acid) |
| Cyanates | Cyanate | ROCN | cyanato- | alkyl cyanate | ||
| Thiocyanate | RSCN | thiocyanato- | alkyl thiocyanate | |||
| Ether | Ether | ROR' | alkoxy- | alkyl alkyl ether | Diethyl ether (Ethoxyethane) | |
| Ester | Ester | RCOOR' | 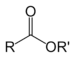 |
-oate | Ethyl butyrate (Ethyl butanoate) | |
| Haloalkane | Halo | RX | halo- | alkyl halide | Chloroethane (Ethyl chloride) | |
| Hydroperoxide (see organic peroxide) | Hydroperoxy | ROOH | hydroperoxy- | alkyl hydroperoxide | Methyl ethyl ketone peroxide | |
| Imine | Primary ketimine | RC(=NH)R' | -skeletal.png) |
imino- | -imine | |
| Secondary ketimine | RC(=NR)R' | -skeletal.png) |
imino- | -imine | ||
| Primary aldimine | RC(=NH)H | -skeletal.png) |
imino- | -imine | ||
| Secondary aldimine | RC(=NR')H | -skeletal.png) |
imino- | -imine | ||
| Isocyanide | Isocyanide | RNC | isocyano- | alkyl isocyanide | ||
| Isocyanates | Isocyanate | RNCO | isocyanato- | alkyl isocyanate | ||
| Isothiocyanate | RNCS | isothiocyanato- | alkyl isothiocyanate | Allyl isothiocyanate | ||
| Ketone | Ketone | RCOR' |  |
keto-, oxo- | -one |  Methyl ethyl ketone (Butanone) |
| Nitrile | Nitrile | RCN | cyano- |
alkanenitrile |
 Benzonitrile (Phenyl cyanide) | |
| Nitro compound | Nitro | RNO2 |  |
nitro- | 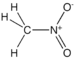 Nitromethane | |
| Nitroso compound | Nitroso | RNO | nitroso- | Nitrosobenzene | ||
| Peroxide | Peroxy | ROOR | 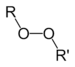 |
peroxy- | alkyl peroxide | Di-tert-butyl peroxide |
| Benzene derivative | Phenyl | RC6H5 | phenyl- | -benzene |  Cumene (2-phenylpropane) | |
| Phosphine | Phosphino | R3P | phosphino- | -phosphane | Methylpropylphosphane | |
| Phosphodiester | Phosphate | HOPO(OR)2 | 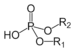 |
phosphoric acid di(substituent) ester | di(substituent) hydrogenphosphate | DNA |
| Phosphonic acid | Phosphono | RP(=O)(OH)2 |  |
phosphono- | substituent phosphonic acid | Benzylphosphonic acid |
| Phosphate | Phosphate | ROP(=O)(OH)2 | 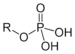 |
phospho- | Glyceraldehyde 3-phosphate | |
| Pyridine derivative | Pyridyl | RC5H4N |
|
4-pyridyl |
-pyridine |  Nicotine |
| Sulfide | RSR' | di(substituent) sulfide | Dimethyl sulfide | |||
| Sulfone | Sulfonyl | RSO2R' |  |
sulfonyl- | di(substituent) sulfone |  Dimethyl sulfone (Methylsulfonylmethane) |
| Sulfonic acid | Sulfo | RSO3H |  |
sulfo- | substituent sulfonic acid | 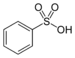 Benzenesulfonic acid |
| Sulfoxide | Sulfinyl | RSOR' |  |
sulfinyl- | di(substituent) sulfoxide |  Diphenyl sulfoxide |
| Thiol | Sulfhydryl | RSH | 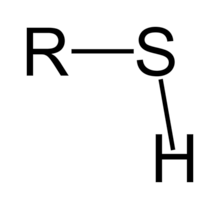 |
mercapto-, sulfanyl- | -thiol | Ethanethiol (Ethyl mercaptan) |
Note: The table above is adapted from the Functional Groups table on Wikipedia.
Combining the names of functional groups with the names of the parent alkanes generates a powerful systematic nomenclature for naming organic compounds.
The non-hydrogen atoms of functional groups are always associated with each by covalent bonds, as well as with the rest of the molecule. When the group of atoms is associated with the rest of the molecule primarily by ionic forces, the group is referred to more properly as a polyatomic ion or complex ion. And all of these are called radicals, by a meaning of the term radical that predates the free radical.
The first carbon after the carbon that attaches to the functional group is called the alpha carbon.
Alcohol containing two hydroxyl groups are called glycols. They have both common names and IUPAC names.
Mnemonics for Functional Groups
These are possible mnemonics for the common functional groups.
Vowels: Remember the vowels "A", "E", and "Y" for Alkane, Alkene, and Alkyne. Alkanes have only single covalent bonds. Alkenes have at least one double bond. Alkynes have at least one triple bond. The letters "I", "O", and "U" are not used. Furthermore, "O" and "U" would result in awkward pronunciations.
Alcohol: Look for the "C-O-H" in "Alcohol."
Ether: Ethers were anesthetics used in the 1800s. Dr. Kellogg also lived at the same time. Corn Flakes are made by Kellogg's. A rooster or cock (C-O-C) is the cornflake mascot. Or, think there is a C on "either" side of the O.
Amine: Remember the "N" stands for nitrogen.
Aldehyde: This sounds like "Adelaide," the Australian city. Australia is at the end of the Asian islands, and aldehydes are at the end of the hydrocarbon chain. The "Y" indicates a C=O double bond.
Ketone: Imagine the diagonal strokes of "K" forming the C=O double bond. ![]()
Carboxylic Acid: "Box" stands for boxed wine or C-O-H, alcohol. The "Y" indicates a C=O double bond.
Ester: This sounds like "Estelle" George Costanza's mother in the TV show Seinfeld. George's nickname was Koko or Coco. So think of O=C-O-C.
Amide: Amine with a "D". D for double.

